载入血管内皮生长因子的肝素化透明质酸大孔水凝胶,用于增强三维内皮细胞迁移和血管形成。
IF 5.5
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2024-10-31
DOI:10.1016/j.bioadv.2024.214094
引用次数: 0
摘要
在巨大的支架内形成强大的血管系统仍然是组织工程领域的一个巨大障碍。人们越来越关注将生物材料支架与多种物理和化学刺激相结合,以增强血管化过程。本研究旨在探讨大孔结构和血管内皮生长因子(VEGF)对细胞迁移和血管化的综合影响。本研究采用明胶微球(GMS)模板浸出法制备了由甲基丙烯酸化透明质酸(HAMA)和甲基丙烯酸化肝素(HepMA)组成的具有不同孔径的肝素化透明质酸(HepHA)大孔水凝胶。在对其物理性质进行表征后,VEGF 被固定在 HepHA 水凝胶上。体外释放研究表明,HepHA 水凝胶可持续释放血管内皮生长因子。随后进行的人脐静脉内皮细胞(HUVECs)迁移评估表明,在具有较大孔隙(VEGF@HepHA250)的 VEGF 负载 HepHA 水凝胶上培养的 HUVECs 迁移得最远。最后,采用背侧皮下模型对水凝胶进行了植入和评估。体内组织学分析结果与体外结果一致,VEGF@HepHA250 水凝胶在植入后四周表现出最明显的血管生成,表明孔隙扩大且富含 VEGF 的水凝胶促进了水凝胶内的血管生成。这项研究揭示了在不同孔径的水凝胶中释放 VEGF 对三维细胞迁移和血管生成的协同效应,从而为设计和制造适于血管生成的组织工程支架提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
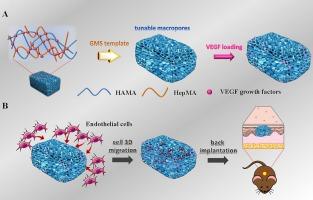
VEGF loading heparinized hyaluronic acid macroporous hydrogels for enhanced 3D endothelial cell migration and vascularization
The formation of robust vascular systems within voluminous scaffolds remains a formidable barrier in the realm of tissue engineering. There is a growing interest in the integration of biomaterial scaffolds with multiple physical and chemical stimuli to augment the process of vascularization. This study aims to investigate the combined impact of macroporous structures and vascular endothelial growth factor (VEGF) on cell migration and vascularization. Heparinized hyaluronic acid (HepHA) macroporous hydrogels with differing pore sizes, composed by methacrylated hyaluronic acid (HAMA) and methacrylated heparin (HepMA), were fabricated by a gelatin microspheres (GMS) template leaching method. After characterization of their physical properties, VEGF was immobilized on the HepHA hydrogels. The in vitro release study indicated that the HepHA hydrogels can provide sustained release of VEGF. Subsequently, cells migration of human umbilical vein endothelial (HUVECs) assessment indicated that HUVECs cultured on VEGF-loaded HepHA hydrogels with larger pores (VEGF@HepHA250) migrated the furthest. Finally, the hydrogels were implanted and evaluated using a dorsal subcutaneous model. The histological analyses conducted in vivo were consistent with the in vitro results, VEGF@HepHA250 hydrogels exhibited the most pronounced vascularization four weeks post-implantation, indicating that hydrogels with expanded pores and an enriched VEGF promoted angiogenesis within the hydrogels. This study sheds light on the synergistic effects of VEGF release on 3D cell migration and vascularization within hydrogels of differing pore sizes, thus providing novel insights into the strategic design and fabrication of tissue-engineered scaffolds that are amenable to vascularization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


