非脂质碱性激动剂强效激活溶血磷脂酸受体 1 的结构机制。
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
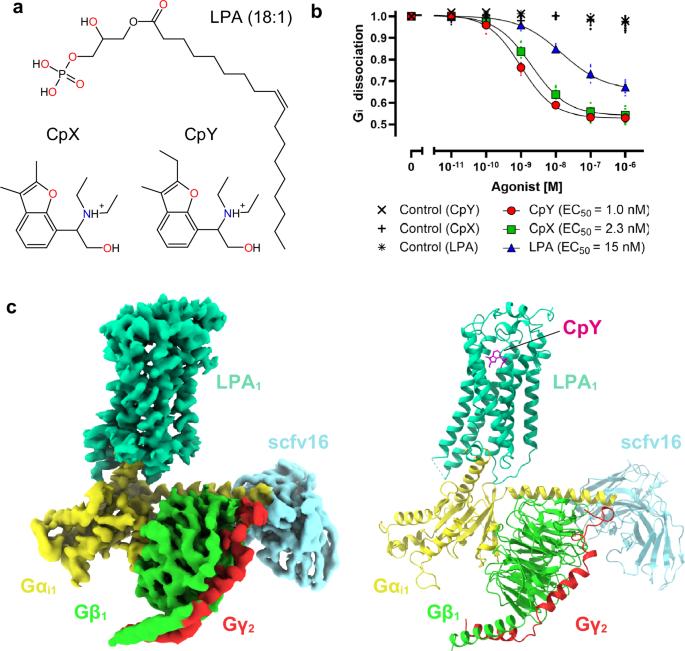
溶血磷脂酸受体 1(LPA1)是由脂质介质溶血磷脂酸(LPA)激活的 G 蛋白偶联受体之一。LPA1 与多种疾病相关,LPA1 激动剂对治疗肥胖症和抑郁症具有潜在的治疗价值。虽然最近发现了强效的非脂质 LPA1 激动剂,但非脂质分子介导的 LPA1 激活机制仍不清楚。在这里,我们报告了与非脂质基本激动剂 CpY 结合的人类 LPA1-Gi 复合物的冷冻电镜结构,与 LPA 相比,CpY 的激动活性高出 30 倍。LPA1 与其他脂质 GPCR 的结构比较显示,LPA1 特性结合口袋中的负电荷允许选择性识别缺乏极性头的 CpY。此外,我们的结构显示,CpY 的乙基直接推动 W2716.48 固定活性构象。内源性 LPA 缺乏这些化学特征,因此这些特征代表了能有效激活 LPA1 的非脂质激动剂的关键要素。这项研究为非脂质激动剂识别配体和激活 LPA1 提供了详细的机理启示,拓展了针对 LPA 受体的药物开发范围。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structural mechanisms of potent lysophosphatidic acid receptor 1 activation by nonlipid basic agonists
Lysophosphatidic acid receptor 1 (LPA1) is one of the G protein-coupled receptors activated by the lipid mediator, lysophosphatidic acid (LPA). LPA1 is associated with a variety of diseases, and LPA1 agonists have potential therapeutic value for treating obesity and depression. Although potent nonlipid LPA1 agonists have recently been identified, the mechanisms of nonlipid molecule-mediated LPA1 activation remain unclear. Here, we report a cryo-electron microscopy structure of the human LPA1-Gi complex bound to a nonlipid basic agonist, CpY, which has 30-fold higher agonistic activity as compared with LPA. Structural comparisons of LPA1 with other lipid GPCRs revealed that the negative charge in the characteristic binding pocket of LPA1 allows the selective recognition of CpY, which lacks a polar head. In addition, our structure show that the ethyl group of CpY directly pushes W2716.48 to fix the active conformation. Endogenous LPA lacks these chemical features, which thus represent the crucial elements of nonlipid agonists that potently activate LPA1. This study provides detailed mechanistic insights into the ligand recognition and activation of LPA1 by nonlipid agonists, expanding the scope for drug development targeting the LPA receptors. A cryo-electron microscopy structure for the human (lysophosphatidic acid receptor 1) LPA1-Gi complex bound to a nonlipid agonist provides mechanistic insights into how nonlipid agonists activate LPA1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


