电针通过调节 Nrf2/HO-1 通路改善糖尿病脑病大鼠的学习和记忆障碍
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
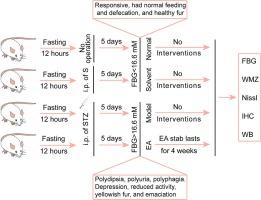
背景:糖尿病脑病(DE)引起的高血糖可导致脑内活性氧过度积累,诱发氧化应激,进而引起神经元变性和凋亡。Nrf2/HO-1信号通路是氧化应激反应中最重要的通路之一,但EA治疗DE的确切机制及其对Nfr2/HO-1通路的具体作用机制仍不清楚:雄性 Wistar 大鼠随机分为四组:正常组、溶剂组、模型组和 EA 组,每组 10 只。腹腔注射链脲佐菌素诱导 DE 大鼠模型。EA 组大鼠双侧刺激 "足三里"(ST36)和 "未央穴"(EXB3),交替进行,每次 30 分钟,每天一次,连续 4 周。用血糖仪测量大鼠的空腹血糖(FBG)水平。用莫里斯水迷宫评估大鼠的学习和记忆能力。通过Nissl染色观察海马CA1区神经元的形态。免疫组化和免疫印迹法检测海马CA1区Nrf2和HO-1的蛋白表达:结果:EA治疗可降低DE大鼠的血糖水平,改善其学习和记忆能力,增加海马CA1区神经元的数量,并上调Nrf2和HO-1的表达。EA治疗可通过调节海马CA1区的Nrf2/HO-1通路抑制氧化应激,对DE大鼠海马区的神经元细胞具有保护作用:结论:EA通过调节海马CA1区的Nrf2/HO-1通路,提高了DE大鼠的学习和记忆能力。这表明 EA 有可能通过减少氧化应激来保护神经元。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electroacupuncture improves learning and memory deficits in diabetic encephalopathy rats by regulating the Nrf2/HO-1 pathway
Background
High blood sugar caused by diabetic encephalopathy(DE) can lead to excessive accumulation of reactive oxygen species in the brain, induce oxidative stress, and subsequently cause neuronal degeneration and apoptosis. The Nrf2/HO-1 signaling pathway is one of the most important pathways in oxidative stress response, but the precise mechanism of EA treatment for DE and its specific mechanism of action on the Nfr2/HO-1 pathway remain unclear.
Methods
Male Wistar rats were randomly assigned to four groups: normal, solvent, model, and EA, with 10 rats per group. A DE rat model was induced by intraperitoneal injection of streptozotocin. EA was applied to stimulate the “Zusanli” (ST36) and “Weiwanxiashu” (EXB3) points bilaterally, alternately for 30 min each, once a day for 4 weeks in the EA group. The rats’ fasting blood glucose(FBG) levels were measured with a glucometer. The Morris water maze was used to evaluate their learning and memory abilities. The morphology of neurons in the CA1 area of the hippocampus was observed by Nissl staining. Detection of protein expression of Nrf2 and HO-1 in the CA1 area of the hippocampus was performed by immunohistochemistry and immunoblotting.
Results
EA treatment reduced blood glucose levels, improved learning and memory abilities, increased the number of neurons in the hippocampal CA1 area, and upregulated the expression of Nrf2 and HO-1 in rats with DE. EA treatment may inhibit oxidative stress by modulating the Nrf2/HO-1 pathway in the hippocampal CA1 area, exerting a protective effect on neuronal cells in the hippocampal area in DE.
Conclusion
EA enhances the learning and memory abilities of rats with DE by regulating the Nrf2/HO-1 pathway in the CA1 area of the hippocampus. This indicates that EA has the potential to protect neurons by reducing oxidative stress.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


