退火有害时:HMGB1 靶向 G-四叉适配体的案例。
IF 7.7
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2024-11-04
DOI:10.1016/j.ijbiomac.2024.137148
引用次数: 0
摘要
在这项工作中,我们介绍了最近发现的用于识别 HMGB1(一种涉及炎症、自身免疫性疾病和癌症的蛋白质)的 G-四重(G4)形成的适配体。这些适配体在没有退火的情况下,在模拟该蛋白质发挥病理活性的细胞外环境的假生理缓冲液中适当稀释后进行了分析,结果表明它们具有很高的热稳定性和抗核酸酶性、良好的蛋白质亲和性和显著的体外活性。这些特征在溶液中形成二聚平行 G4 结构的适配体中更为明显。在此,我们对稀释样品进行标准退火处理后,对相同的抗 HMGB1 合体进行了全面鉴定。值得注意的是,在热展开/折叠循环后,这些适配体,尤其是未退火的最佳适配体,与未退火分析的相同体系相比,出现了明显的构象变化,完全形成了单体 G4 结构,热稳定性和酶稳定性较差,蛋白质亲和力也较低。这些结果证明,对于在所选条件下分析的这些适配体来说,低浓度退火并不会产生有利于生物活性最强的物种的有利影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
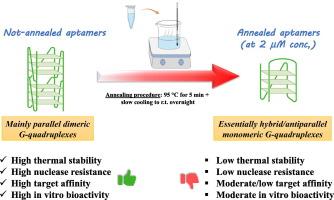
When annealing is detrimental: The case of HMGB1-targeting G-quadruplex aptamers
In this work, we present the case of the G-quadruplex(G4)-forming aptamers we recently identified for the recognition of HMGB1, protein involved in inflammation, autoimmune diseases and cancer. These aptamers were previously analyzed, without annealing them, after proper dilution of the stock solution in a pseudo-physiological buffer mimicking the extracellular environment where the protein exerts its pathological activity, and showed high thermal stability and nuclease resistance, good protein affinity and remarkable in vitro activity. These features were more marked for the aptamers forming dimeric, parallel G4 structures in solution.
Herein, we fully characterized the same anti-HMGB1 aptamers after a standard annealing procedure performed on diluted samples. Notably, upon a thermal unfolding/folding cycle, these aptamers, and particularly the best ones in the not-annealed form, showed significant conformational switches compared to the same systems analyzed without annealing, forming exclusively monomeric G4 structures, featured by poor thermal and enzymatic stabilities, along with lower protein affinities.
These results prove that, for these aptamers, analyzed in the chosen conditions, annealing at low concentration does not produce a beneficial effect in terms of favouring the most bioactive species.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


