双酚 S 暴露通过调节 Gli1 介导的声子刺猬通路促进三阴性乳腺癌细胞的干性。
IF 7.7
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
双酚 S(BPS)是双酚 A(BPA)最常见的替代品之一,被认为会增加罹患乳腺癌的风险。三阴性乳腺癌(TNBC)是一种侵袭性很强的乳腺癌,预后较差。然而,BPS 与 TNBC 之间的关系仍不清楚。癌症干细胞(CSCs)在乳腺癌的发生、转移和复发中起着至关重要的作用。在此,我们提出,相当于人体内部暴露和环境浓度的BPS可通过上调球体形成、自我更新、CD44+/CD24-细胞百分比和CSC标志物的表达来增强CSC样特性。此外,BPS 还能促进 TNBC 细胞的迁移、侵袭和上皮-间质转化(EMT)。从机理上讲,BPS激活了TNBC细胞中的Sonic Hedgehog(SHH)信号通路。分子对接分析进一步表明,BPS通过直接结合Gli1蛋白上调SHH信号通路。此外,SHH通路抑制剂或Gli1 siRNA减弱了BPS对TNBC细胞干性、侵袭和迁移的促进作用。总之,我们的数据首次证明了环境相关浓度的BPS处理可通过激活Sonic Hedgehog/Gli1信号通路显著增强TNBC的恶性表型,引起了人们对BPS潜在群体生物学危害的高度关注。本文章由计算机程序翻译,如有差异,请以英文原文为准。
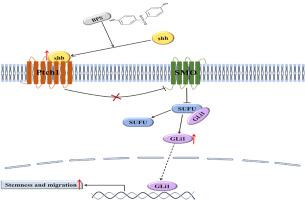
Bisphenol S exposure promotes stemness of triple-negative breast cancer cells via regulating Gli1-mediated Sonic hedgehog pathway
Bisphenol S (BPS), one of the most common alternatives for bisphenol A (BPA), has been implied to increase the risk of breast cancer. Triple-negative breast cancer (TNBC) is a highly aggressive type of breast cancer with a poor prognosis. However, the association between BPS and TNBC remains unclear. Cancer stem cells (CSCs) have a crucial role in breast cancer initiation, metastasis, and recurrence. Here, we proposed that BPS, equivalent to the human internal exposure and the environmental concentrations, enhanced CSC-like properties by upregulating sphere formation, self-renewal, the percentage of CD44+/CD24- cells, and the expression of CSC markers. Moreover, BPS promoted the migration, invasion, and epithelial-mesenchymal transition (EMT) in TNBC cells. Mechanistically, BPS activated the Sonic Hedgehog (SHH) signaling pathway in TNBC cells. Molecular docking analysis further showed that BPS upregulated SHH signaling pathway via directly binding Gli1 protein. Furthermore, inhibitor of SHH pathway or Gli1 siRNA attenuated the promoting effects of BPS on stemness, invasion, and migration of TNBC cells. In summary, our data firstly provide evidence that environmentally relevant BPS concentration treatment significantly enhanced TNBC malignant phenotype by activating the Sonic Hedgehog/Gli1 signaling pathway, raising high concerns about the potential population biology hazards of BPS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Research
环境科学-公共卫生、环境卫生与职业卫生
CiteScore
12.60
自引率
8.40%
发文量
2480
审稿时长
4.7 months
期刊介绍:
The Environmental Research journal presents a broad range of interdisciplinary research, focused on addressing worldwide environmental concerns and featuring innovative findings. Our publication strives to explore relevant anthropogenic issues across various environmental sectors, showcasing practical applications in real-life settings.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


