在极端的 Zn(BF4)2 电解质环境中设计原位杂金属层以实现稳健的锌电化学性能
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
锌金属电池的性能受到来自各种锌盐配方的电解质环境的严重影响。特别是 Zn(BF4)2,它具有显著的成本优势,而且其含氟基团有利于形成有益的 ZnF2 界面层,因此很有应用前景。然而,基于 Zn(BF4)2 的电解质的强酸性会加剧枝晶的形成并促进寄生反应,从而导致电池迅速失效。本文采用 M(BF4)n(M:铜、锡、铟)盐作为 Zn(BF4)2 电解液的添加剂,原位构建异金属层。通过比较,In(BF4)3 衍生的 ZnIn 界面表现出卓越的耐腐蚀能力和最强的锌亲和性,可保护阳极免受酸性侵蚀,并加速 Zn2+ 运输动力学。使用优化电解质的对称电池寿命长达 2500 个循环,而使用聚苯胺阴极的完整电池在 1500 个循环后的容量保持率也高达 81.3%,优于使用原始 Zn(BF4)2 电解质的电池。通过电解质添加剂在电池内生成界面层的策略可随时应用于其他金属电池技术。本文章由计算机程序翻译,如有差异,请以英文原文为准。
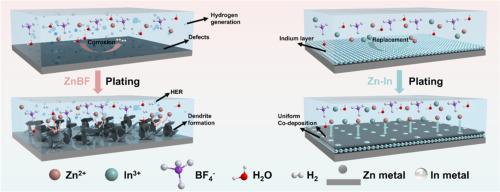
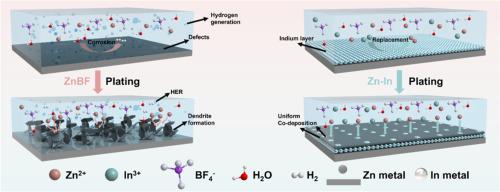
Engineering in situ heterometallic layer for robust Zn electrochemistry in extreme Zn(BF4)2 electrolyte environment
The performance of zinc metal batteries is critically affected by the electrolyte environment originating from various zinc salt formulations. Zn(BF4)2, in particular, offers a notable cost advantage and its fluoride-containing groups facilitate the formation of a beneficial ZnF2 interfacial layer, thereby making it a promising candidate for application. Nonetheless, the strong acidity of the Zn(BF4)2-based electrolyte exacerbates the dendrite formation and promotes parasitic reactions, leading to rapid battery failure. Herein, M(BF4)n (M: Cu, Sn, In) salts were adopted as additives in Zn(BF4)2 electrolyte to in situ construct the heterometallic layers. Through comparison, the In(BF4)3-derived ZnIn interface demonstrates superior corrosion-resistance capability and the strongest zinc affinity, protecting the anode from acidic erosion and accelerating the Zn2+ transportation kinetics. The symmetric cell with the optimized electrolyte exhibits a long lifespan of 2500 cycles while the full cell involving the polyaniline cathode also presents a high capacity retention of 81.3 % after 1500 cycles, outperforming the cell with the original Zn(BF4)2 electrolyte. The strategy of generating an interface layer within the battery through electrolyte additives can be readily applied to other metal battery technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


