通过赖氨酸靶向和新锌螯合抑制突变选择性 AKT
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
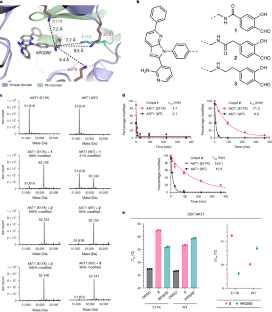
在多种实体瘤中发现了致癌激酶 AKT1 的体细胞改变。最常见的 AKT1 改变是在调节性 pleckstrin 同源结构域1 中将 Glu17 替换为 Lys(E17K),导致构成性膜定位和致癌信号的激活。在临床研究中,发现泛 AKT 抑制剂会引起剂量限制性高血糖2,3,4,5,6,这促使人们寻找突变选择性抑制剂。我们利用 E17K 突变设计了以赖氨酸为靶点的异构水杨醛抑制剂,这种抑制剂对 AKT1 (E17K) 比对野生型 AKT 类似物具有选择性,鉴于异构位点附近存在三个保守赖氨酸,这是一项重大挑战。共价抑制剂复合物的晶体学分析意外地发现了一个不定的四面体锌离子,它能协调激酶活化环中的两个近端半胱氨酸,同时与 E17K-亚胺共轭物结合。与 AKT1 (E17K) 的水杨醛亚胺复合物,而不是与野生型 AKT1 的水杨醛亚胺复合物,会在细胞中募集内源性 Zn2+,从而产生持续的抑制作用。基于水杨醛的抑制剂对 AKT1 (E17K) 肿瘤异种移植模型有效,其剂量不会诱发高血糖。我们的研究表明,通过靶向突变赖氨酸以及水杨醛亚胺加合物产生的 Zn2+ 螯合作用,有可能对 AKT1 (E17K) 实现基于停留时间的精细选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Mutant-selective AKT inhibition through lysine targeting and neo-zinc chelation
Somatic alterations in the oncogenic kinase AKT1 have been identified in a broad spectrum of solid tumours. The most common AKT1 alteration replaces Glu17 with Lys (E17K) in the regulatory pleckstrin homology domain1, resulting in constitutive membrane localization and activation of oncogenic signalling. In clinical studies, pan-AKT inhibitors have been found to cause dose-limiting hyperglycaemia2–6, which has motivated the search for mutant-selective inhibitors. We exploited the E17K mutation to design allosteric, lysine-targeted salicylaldehyde inhibitors with selectivity for AKT1 (E17K) over wild-type AKT paralogues, a major challenge given the presence of three conserved lysines near the allosteric site. Crystallographic analysis of the covalent inhibitor complex unexpectedly revealed an adventitious tetrahedral zinc ion that coordinates two proximal cysteines in the kinase activation loop while simultaneously engaging the E17K–imine conjugate. The salicylaldimine complex with AKT1 (E17K), but not that with wild-type AKT1, recruits endogenous Zn2+ in cells, resulting in sustained inhibition. A salicylaldehyde-based inhibitor was efficacious in AKT1 (E17K) tumour xenograft models at doses that did not induce hyperglycaemia. Our study demonstrates the potential to achieve exquisite residence-time-based selectivity for AKT1 (E17K) by targeting the mutant lysine together with Zn2+ chelation by the resulting salicylaldimine adduct. A mutant-selective AKT inhibitor shows potential as a targeted therapy for breast cancer, enabling enhanced target engagement and avoiding the dose-limiting toxicity associated with pan-AKT inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


