探索共价的界限
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
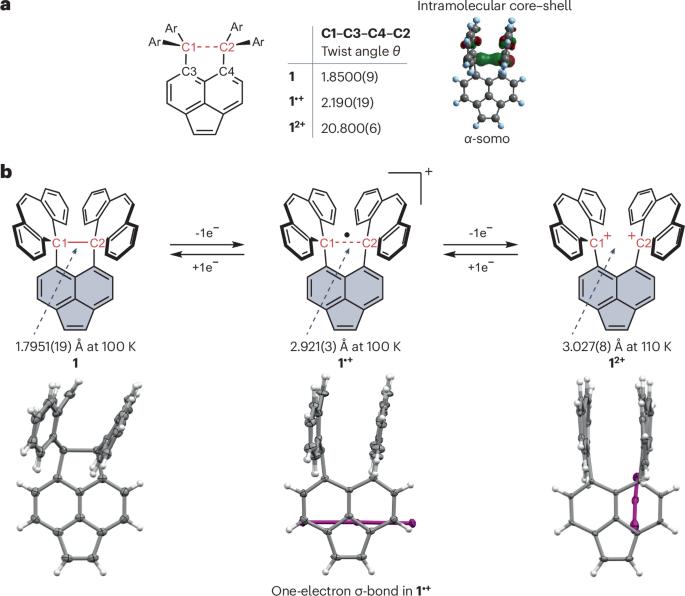
单电子σ键于 1931 年首次提出,但此后的讨论大多停留在理论层面。首次在实验中观察到的单电子 C-C 键既增进了我们对键的基本了解,又为创造一类新分子提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the boundaries of covalency
One electron σ-bonds were first proposed in 1931 but most discussion since then has been at a theoretical level. The first experimentally observed single-electron C–C bond both advances our fundamental understanding of bonding and provides the basis of an approach to creating a new class of molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


