根据自人工模板剂的热解机理调节二氧化碳吸附剂的性能
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
以往的研究证明,吸附剂的孔隙形状和氮基团含量对其二氧化碳(CO2)吸附性能起着至关重要的作用。本文以壳聚糖为碳源,氮化碳(g-C3N4 和 g-C3N5)为自吸收模板剂,KOH 为活化剂,通过改变氮化碳的比例、热解温度和 KOH 的活化比,制备了一系列掺氮多孔碳材料,用于吸附二氧化碳。在制备的材料中,T6-850-1 的比表面积(SBET)最高,为 2336 m2/g;T6-750-1 的微孔面积(Smicro)和二氧化碳吸附容量(1 bar, 298 K)最高,分别为 1969 m2/g 和 3.49 mmol/g。通过热重红外气相色谱-质谱法(TG-IR-GC-MS)对氮化碳模板的热分解温度和产物进行了表征和测试,并研究了两种氮化碳模板的热分解机理。我们发现,模板的热稳定性直接影响最终样品的孔结构以及氮物种的类型和数量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
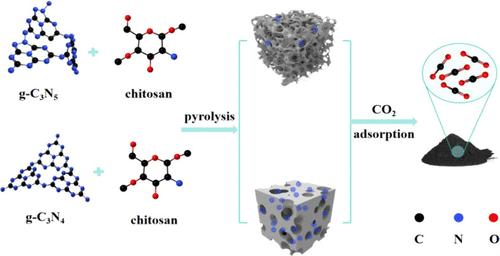
Regulating the Performance of CO2 Adsorbents Based on the Pyrolysis Mechanism of Self-Sacrificial Templating Agents
Previous research has proven that the pore shape and nitrogen group content of adsorbents play essential roles in determining their carbon dioxide (CO2) adsorption performance. In this article, a series of nitrogen-doped porous carbon materials were prepared for CO2 adsorption by varying the proportion of carbon nitride, the pyrolysis temperature, and the activation ratio of KOH, using chitosan as the carbon source, carbon nitride (g-C3N4 and g-C3N5) as self-sacrificing templating agents, and KOH as the activator. Among the prepared materials, T6-850-1 has the highest specific surface area (SBET) of 2336 m2/g, and T6-750-1 has the highest microporous area (Smicro) and CO2 adsorption capacity (1 bar, 298 K) of 1969 m2/g and 3.49 mmol/g, respectively. The thermal decomposition temperature and products of carbon nitride templates were characterized and tested by thermogravimetric infrared gas chromatography-mass spectrometry (TG-IR-GC-MS), and the thermal decomposition mechanisms of the two carbon nitride templates were investigated. We found that the thermal stability of the template directly affects the pore structure of the final sample as well as the type and quantity of nitrogen species.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


