缺氧诱导癌症炎症细胞死亡的机制
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
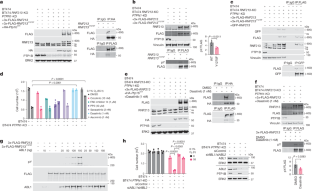
缺氧状态下的癌细胞会抵制多种抗肿瘤疗法,并可能导致复发1,2。RNF213是一种具有多个AAA-ATPase结构域和两个泛素连接酶结构域(RING和RZ)的大蛋白,与莫亚莫亚病、脂肪毒性和先天免疫有关4。我们在此报告 PTP1B 和 ABL1/2 相互控制 RNF213 的酪氨酸磷酸化,进而控制其寡聚化和 RZ 结构域的激活。RZ 结构域泛素化并诱导主要 NF-κB 调节因子 CYLD/SPATA2 降解。CYLD/SPATA2水平的降低会导致NF-κB激活并诱导NLRP3炎性体,而NLRP3炎性体与缺氧诱导的内质网应激一起,会引发细胞热解死亡。与这一模型相一致的是,CYLD 基因缺失会复制 PTP1B 基因缺失对人类表皮生长因子受体 2 阳性乳腺癌异种移植生长的影响,而 NLRP3 基因缺失则会阻止这种影响。用 RNF213 突变体进行的重组研究证实,RZ 结构域介导了肿瘤细胞的死亡。总之,我们的研究结果确定了一种独特的、可能成为靶点的 PTP1B-RNF213-CYLD-SPATA2 通路,它对控制缺氧肿瘤中的炎症细胞死亡至关重要,为 RNF213 的调控提供了新的见解,并对莫亚莫亚病、炎症性疾病和自身免疫性疾病的发病机制具有潜在的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。

A mechanism for hypoxia-induced inflammatory cell death in cancer
Hypoxic cancer cells resist many antineoplastic therapies and can seed recurrence1,2. We previously found that either deficiency or inhibition of protein-tyrosine phosphatase (PTP1B) promotes human epidermal growth factor receptor 2-positive breast cancer cell death in hypoxia by activation of RNF213 (ref. 3), a large protein with multiple AAA-ATPase domains and two ubiquitin ligase domains (RING and RZ) implicated in Moyamoya disease, lipotoxicity and innate immunity4. Here we report that PTP1B and ABL1/2 reciprocally control RNF213 tyrosine phosphorylation and, consequently, its oligomerization and RZ domain activation. The RZ domain ubiquitylates and induces the degradation of the major NF-κB regulator CYLD/SPATA2. Decreased CYLD/SPATA2 levels lead to NF-κB activation and induction of the NLRP3 inflammasome which, together with hypoxia-induced endoplasmic reticulum stress, triggers pyroptotic cell death. Consistent with this model, CYLD deletion phenocopies, whereas NLRP3 deletion blocks, the effects of PTP1B deficiency on human epidermal growth factor receptor 2-positive breast cancer xenograft growth. Reconstitution studies with RNF213 mutants confirm that the RZ domain mediates tumour cell death. In concert, our results identify a unique, potentially targetable PTP1B–RNF213–CYLD–SPATA2 pathway critical for the control of inflammatory cell death in hypoxic tumours, provide new insights into RNF213 regulation and have potential implications for the pathogenesis of Moyamoya disease, inflammatory disorders and autoimmune disease. We find that PTP1B and ABL1/2 reciprocally control RNF213 tyrosine phosphorylation and its oligomerization and RZ domain activation, and identify a unique PTP1B–RNF213–CYLD–SPATA2 pathway critical for the control of inflammatory cell death in hypoxic tumours.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


