PdAu12 催化剂中的钯掺杂剂在对取代苄醇氧化过程中的底物依赖性作用:促进氢化物萃取和 O2 的还原活化
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
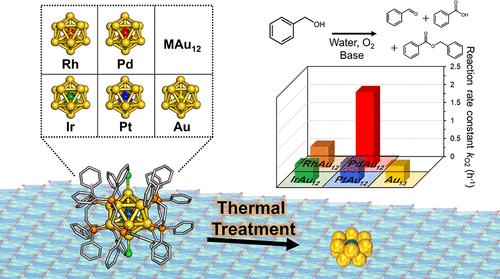
在原子尺寸可控的金属纳米团簇上进行单原子掺杂是实现多金属化的首要步骤,这有助于阐明掺杂对催化作用的影响。在此,我们通过热处理二膦保护的 MAu12(dppe)5Cl2(dppe:1,2-双(二苯基膦)乙烷),在由 Co 和 Ce 组成的双金属氢氧化物上合成了 MAu12(M = Au、Ir、Rh、Pt 或 Pd)纳米团簇。X 射线吸收精细结构光谱和像差校正高角度环形暗场扫描透射电子显微镜证实,在优化的热处理条件(175 °C,8 小时)下成功形成了 MAu12 纳米团簇。在五种催化剂中,PdAu12 在水相苯甲醇(BnOH)氧化中的活性是 Au13 的 4.4 倍,而其他 MAu12(M = Ir、Rh 或 Pt)催化剂的活性与 Au13 相当。根据对取代的 BnOH(R-BnOH;R = MeO、Me、H、Cl 或 CF3)在不同 O2 分压下的动力学实验,我们得出结论:根据 R 基团的性质,单个掺杂钯原子在氧化过程中起着双重作用:R = Cl 或 CF3 时,从吸附的氧化烷中抽取 H-;R = MeO、Me 或 H 时,还原活化 O2。对模型结构的理论研究表明,与 Au13 相比,PdAu12 能更有效地活化 O2,因此掺杂单一 Pd 时,活化的 O2 会出现不同的机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Substrate-Dependent Role of a Pd Dopant in PdAu12 Catalysts in the Oxidation of p-Substituted Benzyl Alcohols: Promotion of Hydride Abstraction and Reductive Activation of O2
Single-atom doping, which is the primary step toward multimetallization, on atomically size-controlled metal nanoclusters facilitates the elucidation of doping effects on catalysis. Herein, we synthesized MAu12 (M = Au, Ir, Rh, Pt, or Pd) nanoclusters on double metal hydroxide composed of Co and Ce by thermal treatment of diphosphine-protected MAu12(dppe)5Cl2 (dppe: 1,2-bis(diphenylphosphino)ethane). The successful formation of the MAu12 nanocluster under an optimized thermal treatment condition (175 °C, 8 h) was confirmed by X-ray absorption fine structure spectroscopy and aberration-corrected high-angle annular dark-field scanning transmission electron microscopy. Among the five catalysts, PdAu12 exhibited 4.4 times higher activity than Au13 in the aqueous phase benzyl alcohol (BnOH) oxidation, while the other MAu12 (M = Ir, Rh, or Pt) catalysts showed comparable activity to Au13. On the basis of the kinetic experiments under different partial pressures of O2 with p-substituted BnOH (R-BnOH; R = MeO, Me, H, Cl, or CF3), we concluded that the single Pd atom dopant plays a dual role in the oxidation depending on the nature of the R group: abstraction of H– from the adsorbed alkoxide for R = Cl or CF3 and reductive activation of O2 for R = MeO, Me, or H. Theoretical studies on model structures have shown that O2 is more efficiently activated by PdAu12 than by Au13, thereby the different mechanism mediated by activated O2 appears with the single Pd doping.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


