远紫外光分解硝酸盐产生的活性氮物种有助于农药降解和含氮副产物的形成
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
气候变化导致农业中农药和化肥的使用量增加,导致接受农业径流的水生生态系统中农药和硝酸盐含量升高。在这项研究中,我们证明了远紫外线(UV222)光解硝酸盐可快速降解地表水中的四种农药,其降解率常数是 UV254 光解硝酸盐的 37.1-144.75 倍。农药降解率的提高不仅是因为 UV222 的直接光解作用比 UV254 强,还因为在 UV222/硝酸盐过程中产生的羟基自由基(HO-)和活性氮物种(如 NO2- 和 ONOO-)增多。我们确定了 222 纳米波长下硝酸盐光解的先天量子产率,并将这些值纳入动力学模型,从而可以准确预测硝酸盐的光降解和活性物种的生成。在 UV222/硝酸盐过程中,反应性氮物种主要导致农药降解,但它们也会形成硝化副产物。利用稳定同位素标记的硝酸盐(15NO3-)结合质谱分析,我们证实硝化副产物是由硝酸盐光解产生的活性氮物种形成的。此外,我们还证明,在实际地表水中进行后氯化处理时,UV222/硝酸盐过程会增加剧毒含氮氯化产物(如三氯硝基甲烷)形成的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
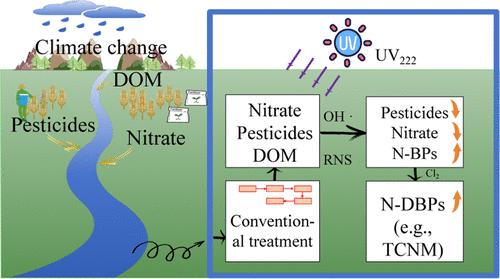
Reactive Nitrogen Species Generated from Far-UVC Photolysis of Nitrate Contribute to Pesticide Degradation and Nitrogenous Byproduct Formation
Climate change has resulted in increased use of pesticides and fertilizers in agriculture, leading to elevated pesticide and nitrate levels in aquatic ecosystems that receive agricultural runoff. In this study, we demonstrate that far-UVC (UV222) photolysis of nitrate rapidly degrades four pesticides in surface water, with a degradation rate constant 37.1–144.75 times higher than that achieved by UV254 photolysis of nitrate. The improved pesticide degradation is due not only to the enhanced direct photolysis by UV222 compared to UV254 but also to the increased generation of hydroxyl radicals (HO•) and reactive nitrogen species (e.g., NO2• and ONOO–) in the UV222/nitrate process. We determined the innate quantum yields of nitrate photolysis at 222 nm and incorporated these values into a kinetic model, allowing for the accurate prediction of nitrate photodecay and reactive species generation. While reactive nitrogen species predominantly contribute to pesticide degradation in the UV222/nitrate process, they also lead to the formation of nitration byproducts. Using stable isotope-labeled nitrate (15NO3–) combined with mass spectrometry, we confirmed that the nitration byproducts are formed from the reactive nitrogen species generated from nitrate photolysis. Additionally, we demonstrate that the UV222/nitrate process increases the formation potential of highly toxic nitrogenous chlorinated products (e.g., trichloronitromethane) during postchlorination in real surface water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


