二维过氧化物有机层中的链式熔融温度降低
IF 18.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
含有正烷基铵阳离子的二维(2D)包晶往往会在压力或温度变化时发生固-固相变,其间有机阳离子会发生有序到无序的链熔化转变。在这里,我们展示了混合不同长度的卤化物和有机阳离子会降低铜基和锰基二维包晶的相变温度。这种降低的幅度取决于两个正烷基铵阳离子的相对长度。例如,将正癸基铵(DA)与正十一烷基铵(UDA)混合,相变温度会比纯 DA2CuBr4 降低 5 °C。相比之下,将 DA 与正十二烷基铵 (DDA) 混合会导致相变温度降低 25 °C。此外,我们还发现,由于 DA2CuCl4 和 DA2MnCl4 中无机层之间的晶格间距相似,掺入 Cu2+ 和 Mn2+ 对相变温度的影响微乎其微。我们的研究为通过合金化卤化物、有机阳离子和金属阳离子来调整二维包晶石的结构相变提出了新的设计原则。本文章由计算机程序翻译,如有差异,请以英文原文为准。
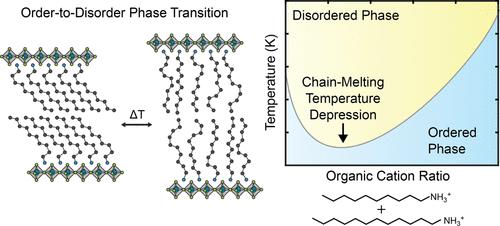
Chain-Melting Temperature Depression in the Organic Layer of Two-Dimensional Perovskites
Two-dimensional (2D) perovskites with n-alkylammonium cations often undergo a solid–solid phase transition upon changes in pressure or temperature, during which the organic cations undergo an order-to-disorder chain-melting transition. Here, we show that blending halides and organic cations of varying lengths decreases the phase transition temperature of Cu- and Mn-based 2D perovskites. The magnitude of this decrease depends on the relative lengths of the two n-alkylammonium cations. For instance, blending n-decylammonium (DA) with n-undecylammonium (UDA) results in a 5 °C decrease in the phase transition temperature compared to pure DA2CuBr4. In contrast, blending DA with n-dodecylammonium (DDA) causes a 25 °C decrease. Furthermore, we found that blending Cu2+ and Mn2+ has a minimal effect on the phase transition temperature due to similar lattice spacing between the inorganic layers in DA2CuCl4 and DA2MnCl4. Our study suggests new design principles for tuning structural phase transitions in 2D perovskites through alloying halides, organic cations, and metal cations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


