组织中 COVID-19 疫苗免疫记忆的维持和功能调节
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
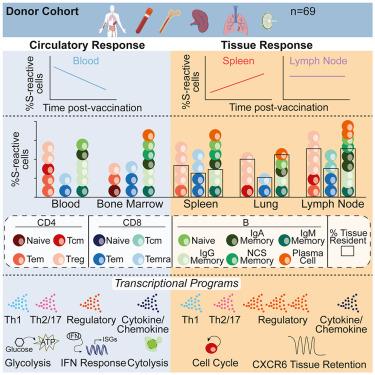
组织中的记忆性 T 细胞和 B 细胞对保护性免疫至关重要。在这里,我们对 COVID-19 mRNA 疫苗诱导的免疫记忆在血液、淋巴器官和肺部的组织分布、表型、持久性和转录谱进行了全面分析,研究对象是 63 名年龄在 23-86 岁之间的器官捐献者,其中一些人曾感染过 SARS-CoV-2。在淋巴器官和肺部检测到尖峰(S)反应记忆T细胞,并根据感染史不同表达不同的组织驻留标记,S反应B细胞包括驻留在淋巴器官的类调换记忆细胞。与血液相比,S反应性组织记忆T细胞在接种疫苗后持续存在的时间更长,而且随着年龄的增长更为普遍。S 反应性 T 细胞显示出特定部位的亚群组成和功能:调节细胞特征在组织中更为丰富,而效应细胞和细胞溶解特征在循环中更为丰富。我们的研究结果揭示了疫苗诱导的 T 细胞记忆的功能分区,在这些分区中,监测效应细胞和原位调节反应可提供保护,同时将组织损伤降到最低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Maintenance and functional regulation of immune memory to COVID-19 vaccines in tissues
Memory T and B cells in tissues are essential for protective immunity. Here, we performed a comprehensive analysis of the tissue distribution, phenotype, durability, and transcriptional profile of COVID-19 mRNA vaccine-induced immune memory across blood, lymphoid organs, and lungs obtained from 63 vaccinated organ donors aged 23–86, some of whom experienced SARS-CoV-2 infection. Spike (S)-reactive memory T cells were detected in lymphoid organs and lungs and variably expressed tissue-resident markers based on infection history, and S-reactive B cells comprised class-switched memory cells resident in lymphoid organs. Compared with blood, S-reactive tissue memory T cells persisted for longer times post-vaccination and were more prevalent with age. S-reactive T cells displayed site-specific subset compositions and functions: regulatory cell profiles were enriched in tissues, while effector and cytolytic profiles were more abundant in circulation. Our findings reveal functional compartmentalization of vaccine-induced T cell memory where surveilling effectors and in situ regulatory responses confer protection with minimal tissue damage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


