碱性电解质下的水质子电池
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
水质质子电池(APB)因其快速的动力学、低成本和可持续性而备受关注。然而,大多数报道的水质子电池都是在酸性条件下工作的,这将不可避免地导致严重的氢进化反应(HER)和腐蚀问题。在此,我们探索了一种含有偶氮基团(N=N)的小分子有机化合物偶氮苯(AB),将其作为碱性电解质下的 APB 阳极材料,其氧化还原电位约为 -0.6 V(相对于 Hg/HgO)。AB 阳极在 10 A g-1 的高电流密度下实现了 151 mAh g-1 的高倍率容量,并在 2 M KOH 电解液下实现了 12,500 次循环的出色稳定性。实验结果和理论计算证实,AB 发生了双电子氧化还原反应,涉及一个中心 N=N 基团与来自 H2O 分子的两个质子。这项研究揭示了偶氮材料中的质子化现象,为设计先进质子二次电池中具有多电子氧化还原反应的有机小分子提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
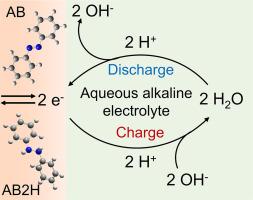
An aqueous proton battery under alkaline electrolyte
Aqueous proton batteries (APBs) have attracted much attention owing to their fast kinetics, low cost and sustainability. However, the most reported APBs operate under acidic conditions, which will lead to serious hydrogen evolution reaction (HER) and corrosion problem, inevitably. Here, a small molecule organic compound, azobenzene (AB) containing an azo group (N = N), is explored as an anode material for APBs under alkaline electrolyte with a low redox potential of around -0.6 V (vs. Hg/HgO). AB anode achieves high-rate capacity of 151 mAh g-1 at a high current density of 10 A g-1 and excellent cycling stability over 12,500 cycles under 2 M KOH electrolyte. The experimental results and theoretical calculations confirm that AB performs a two-electron redox reaction involving a central N = N group with two protons from H2O molecules. This work reveals the protonation in azo-materials and promotes new insights on design small organic molecules with multi-electron redox reactions in advanced proton secondary batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


