通过在原本绝缘的 Na6ZnS4 中进行间隙位点工程创建传导通道,实现 Na 导电固态电解质
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
Na5.6Zn0.6Ga0.4S4 保留了其母体形式 Na6ZnS4 的晶体结构,被描述为一种用于全固态电池的新型 Na 导电固态电解质 (SSE)。我们证明,虽然 Na6ZnS4 在离子上是绝缘的(1.4 nS cm-1),但取代 Ga 后,离子电导率(σion)惊人地提高到 70.1 μS cm-1,使 Na5.6Zn0.6Ga0.4S4 成为一种实用的固态电解质。σ离子的这种急剧增加(5 × 104 倍)与 Na6-xZn1-xGaxS4 中 "x "增加时间隙位点的 Na+ 占有率增加有关,间隙 Na 离子促进了长程 Na+ 传导,否则 Na+ 是不动的。镓取代也会导致 Na6-xZn1-xGaxS4 的相纯,至少部分地增强了σ离子。此外,Na6-xZn1-xGaxS4 在环境条件下几个小时既不会释放出 H2S 气体,也不会破坏其晶体结构。镓的取代也增强了电化学稳定性。虽然阳极极限基本保持不变,但阴极极限却从 Na6ZnS4 的 0.99 V 对 Na2Sn 显著降低到 Na5.6Zn0.6Ga0.4S4 的 0.35 V,从而在对称的 Na2Sn ‖ Na2Sn 电池中实现了稳定的 Na 合金/合金化反应。这些发现得到了各种实验和理论方法的全面支持。最后,我们构建了一个完整的电池(Na2Sn ‖ TiS2),并证明了 Na5.6Zn0.6Ga0.4S4 作为所有固态氦离子电池中一种有前途的固态氦离子电池的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
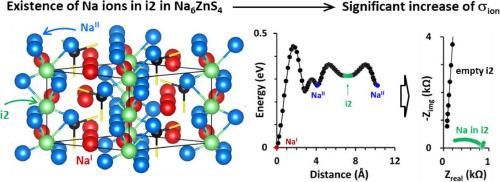
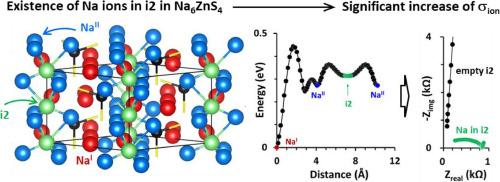
Conduction channel creation by interstitial site engineering in otherwise insulating Na6ZnS4 for Na-conducting solid-state electrolytes
Na5.6Zn0.6Ga0.4S4, which retains the crystalline structure of its parent form Na6ZnS4, is described as a new class of Na-conducting solid-state electrolytes (SSEs) for all-solid-state batteries. We demonstrate that while Na6ZnS4 is ionically insulating (1.4 nS cm-1), Ga-substitution results in an astonishing improvement of ionic conductivity (σion) to 70.1 μS cm-1, making Na5.6Zn0.6Ga0.4S4 a practical SSE. This dramatic increase in σion (5 × 104 fold) is associated with an increased Na+ occupancy in interstitial sites as ‘x’ increases in Na6-xZn1-xGaxS4, where interstitial Na ions facilitate long-range Na+ conduction, which is otherwise immobile. Ga-substitution also results in phase-pure Na6-xZn1-xGaxS4, contributing at least partially to the enhancement of σion. Furthermore, Na6-xZn1-xGaxS4 exhibits neither releasing H2S gas nor compromising its crystalline structure for several hours under ambient conditions. Ga-substitution also enhances electrochemical stability. While the anodic limit remains largely unchanged, the cathodic limit is significantly lowered from 0.99 V vs. Na2Sn in Na6ZnS4 to 0.35 V in Na5.6Zn0.6Ga0.4S4, resulting in stable Na alloying/dealloying reactions in a symmetric Na2Sn ‖ Na2Sn cell. These findings are comprehensively supported by various experimental and theoretical methods. Finally, we construct a full cell (Na2Sn ‖ TiS2) and demonstrate the practicality of Na5.6Zn0.6Ga0.4S4 as a promising SSE in all solid-state Na ion batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


