保持距离:通过可调间隔长度的刚性α-螺旋连接体控制固体支持的蔗糖磷酸化酶的活性
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
将酶固定到载体材料中在生物技术中具有广泛的重要性,然而了解与固体表面结合的酶的催化作用仍然具有挑战性。在这里,我们通过融合蛋白的方法来探索表面对蔗糖磷酸化酶催化作用的影响。我们通过结构上刚性的 α 螺旋连接体 [EA3K]n固定酶,由于使用的五肽重复序列数量可变(n = 6、14、19),所以间隔长度可调。分子建模和模拟方法划定了每个连接体相对于其用于表面系链的 His 标签帽的构象空间。随着间隔物长度的增加,连接体构象的群体分布变得更广,酶到表面的距离也随之变大(≤15 nm)。根据温度动力学研究,我们获得了对溶液中酶与连接体融合的催化作用的能量描述,并将其固定在镍2+螯合琼脂糖上。在不变的吉布斯活化自由能(ΔG‡ = ∼61 kJ/mol,30 °C)框架内,固体支持的酶涉及焓-熵分配的明显变化。对ΔG‡的熵贡献(-TΔS‡)随着间隔长度的增加而增加,从无连接体酶的-16.4 kJ/mol增加到[EA3K]19连接融合的+7.9 kJ/mol。固定的[EA3K]19融合蛋白的催化特性与溶液中的酶没有区别,无论链接器如何,它们的行为都是相同的。可以说,更靠近表面的酶具有更高的分子组织程度("僵化"),必须通过吸收额外的热量来放松以进行催化,并通过熵的增加来补偿。这些酶的热稳定性提高(高达 2.8 倍)与所提出的僵化效应是一致的。总之,我们的研究揭示了表面对蔗糖磷酸化酶催化活化参数的影响,并显示了它们对表面系链连接体长度的一致依赖性。本研究获得的基本见解,以及将这一原理成功推广到另一种酶(蔗糖磷酸化酶)的结果表明,基于刚性连接体的蛋白质表面距离控制可作为一种工程策略,用于优化固定化酶的活性特征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
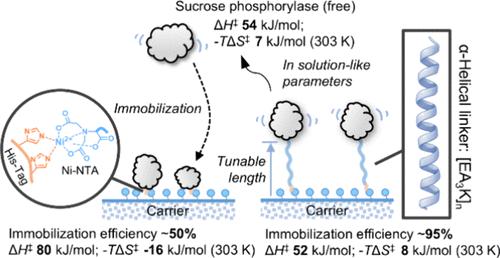
Keeping the Distance: Activity Control in Solid-Supported Sucrose Phosphorylase by a Rigid α-Helical Linker of Tunable Spacer Length
Enzyme immobilization into carrier materials has broad importance in biotechnology, yet understanding the catalysis of enzymes bound to solid surfaces remains challenging. Here, we explore surface effects on the catalysis of sucrose phosphorylase through a fusion protein approach. We immobilize the enzyme via a structurally rigid α-helical linker [EA3K]n of tunable spacer length due to the variable number of pentapeptide repeats used (n = 6, 14, 19). Molecular modeling and simulation approaches delineate the conformational space sampled by each linker relative to its His-tag cap used for surface tethering. The population distribution of linker conformers gets broader, with a consequent shift of the enzyme-to-surface distance to larger values (≤15 nm), as the spacer length increases. Based on temperature kinetic studies, we obtain an energetic description of catalysis by the enzyme-to-linker fusions in solution and immobilize on Ni2+-chelate agarose. The solid-supported enzymes involve distinct changes in enthalpy–entropy partitioning within the frame of invariant Gibbs free energy of activation (ΔG‡ = ∼61 kJ/mol at 30 °C). The entropic contribution (−TΔS‡) to ΔG‡ increases with the spacer length, from −16.4 kJ/mol in the linker-free enzyme to +7.9 kJ/mol in the [EA3K]19 linked fusion. The immobilized [EA3K]19 fusion protein is indistinguishable in its catalytic properties from the enzymes in solution, which behave identically regardless of their linker. Enzymes positioned closer to the surface arguably experience a higher degree of molecular organization (“rigidification”) that must relax for catalysis through the additional uptake of heat, compensated by a gain in entropy. Increased thermostability of these enzymes (up to 2.8-fold) is consistent with the proposed rigidification effect. Collectively, our study reveals surface effects on the activation parameters of sucrose phosphorylase catalysis and shows their consistent dependence on the length of the surface-tethering linker. The fundamental insight here obtained, together with the successful extension of the principle to a different enzyme (nigerose phosphorylase), suggests that rigid linker-based control of the protein–surface distance can be used as an engineering strategy to optimize the activity characteristics of immobilized enzymes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


