新型吡唑并嘧啶作为 p38α 抑制剂和细胞凋亡诱导剂对肾细胞癌(UO-31 细胞)的设计、合成和细胞毒性筛选
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
研究人员设计并合成了一系列以吡唑嘧啶-4-胺为核心的新型分子,这些分子对肾细胞癌细胞(UO-31)具有潜在的细胞毒性。对 UO-31 细胞的细胞毒活性研究结果表明,吡唑嘧啶 19 和 31 的细胞毒活性高于索拉非尼(SOR)。这些化合物的细胞毒性活性似乎与它们抑制 p38α MAPK 的能力有关,其抑制能力分别比 SOR 强 2.53 倍和 2.27 倍。此外,细胞周期分析结果以及附件素-V 对(UO-31)细胞的检测结果表明,吡唑并嘧啶 19 和 31 的促凋亡活性分别比 SOR 高 1.42 倍和 1.20 倍。此外,还发现化合物 19 和 31 能有效阻止细胞周期在 G2/M 期的积累。最后,受试化合物降低了 TNF 浓度,并增加了肿瘤抑制基因 p53、Bax/BCL-2 比率和 caspase 3/7 的表达。本文章由计算机程序翻译,如有差异,请以英文原文为准。
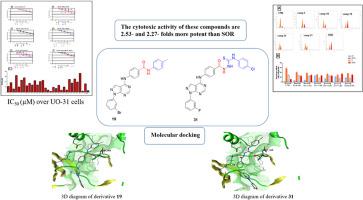
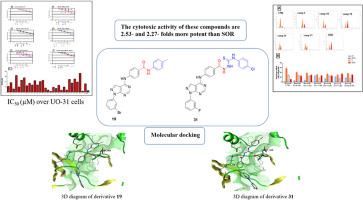
Design, synthesis, and cytotoxicity screening of novel pyrazolopyrimidines over renal cell carcinoma (UO-31 cells) as p38α inhibitors, and apoptotic cells inducing activities
A series of novel molecules with pyrazolopyrimidine-4-amine core were designed and synthesized as potential cytotoxic agents over Renal Cell Carcinoma cells (UO-31). Results of cytotoxic activity against UO-31 cells showed that pyrazolopyrimidines 19 and 31 were found to be more cytotoxic than sorafenib (SOR). The cytotoxic activity of these compounds appeared to correlate with their ability to inhibit p38α MAPK which are 2.53- and 2.27- folds more potent than SOR. Moreover, results of the cell cycle analysis as well as the results of annexin-V on the (UO-31) cells showed that pyrazolopyrimidines 19 and 31 had a pro-apoptotic activity higher than SOR by 1.42- and 1.20- folds, respectively. Furthermore, compounds 19 and 31 were found to be effective in arresting the cell cycle throughout the accumulation of the cells at G2/M phase. Finally, the tested compounds decreased the TNF concentration as well as increased the expression of tumor suppressor gene p53, Bax/BCL-2 ratio and caspase 3/7.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


