烯基硼酸与丙炔基甲磺酸酯 sp3 碳亲电体的无过渡金属亲核取代反应
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种用途广泛的无过渡金属铃木型交叉偶联方案。在温和的碱存在下,烯基/芳基硼酸能与丙炔基甲磺酸酯 Csp3 亲电体顺利偶联。碱中的反离子对反应活性起着至关重要的作用。我们的无金属条件与经典的过渡金属催化的铃木反应是正交的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
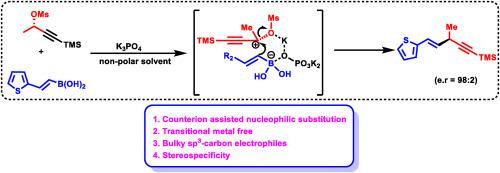
Transition-metal-free nucleophilic substitution of alkenyl boronic acids with propargylic mesylates sp3-carbon electrophiles
We developed a highly versatile transition metal-free Suzuki type cross-coupling protocol. Alkenyl/aryl boronic acids could couple smoothly with propargylic mesylates Csp3 electrophiles in the presence of mild bases. The counterion in the base plays a crucial role for the reactivity. Our metal-free condition is orthogonal towards the classic transition metal catalysed Suzuki reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


