细胞色素 c 与癌细胞代谢:新视角
IF 3.4
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
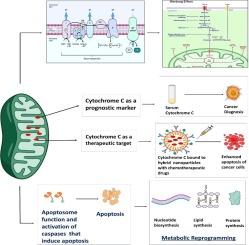
细胞色素 c 是线粒体呼吸链中的重要电子载体。当线粒体外膜发生渗透时,细胞色素 c 就会被释放到细胞质中,从而启动内在凋亡途径。细胞色素 c 与凋亡蛋白酶激活因子-1(Apaf-1)之间的复杂相互作用导致凋亡小体的形成和 caspases 级联反应的激活,从而凸显了细胞色素 c 在控制细胞死亡机制中的关键作用。此外,细胞色素 c 还会发生翻译后修饰,特别是磷酸化,从而错综复杂地调节其在呼吸和细胞凋亡中的作用。这些修饰使细胞色素 c 如何有效控制细胞功能变得更加复杂。细胞色素 c 成为癌症复杂网络中的灯塔,其表达模式为预后和治疗提供了提示。在癌症组织中观察到细胞色素 c 水平降低,这表明细胞凋亡可能受到抑制。例如,在胶质瘤组织中,细胞色素 c 的水平比健康组织低,这种降低在疾病晚期更为明显。然而,细胞色素 c 在癌症中的作用变得更加复杂,因为它与癌细胞的代谢重编程交织在一起。这表明,细胞色素 c 在肿瘤进展和抗药性方面起着至关重要的作用。将细胞色素 c 看作分子马赛克揭示了它不仅仅是一种蛋白质,还是决定细胞命运的核心角色。这一认识为癌症诊断和治疗策略的潜在进步开辟了令人兴奋的途径。尽管取得了进展,但围绕细胞色素 c 的叙述仍不完整,这就需要进一步探索其复杂性以及与癌症相关的生物学意义。细胞色素 c 是抗击癌症的希望灯塔和有前途的治疗目标,特别是因为它在肿瘤代谢中的重要作用。在未来,细胞色素 c 具有开发创新解决方案的潜力,以应对细胞命运的复杂挑战。在这篇综述中,我们试图阐明细胞色素 c 的多面性,并在癌症的背景下将细胞凋亡、新陈代谢重编程和沃伯格效应联系起来。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cytochrome c and cancer cell metabolism: A new perspective
Cytochrome c is a vital electron carrier in the mitochondrial respiratory chain. When the outer membrane of mitochondria becomes permeable, cytochrome c is discharged into the cytoplasm, where it initiates the intrinsic apoptosis pathway. The complex interaction between cytochrome c and apoptosis protease-activating factor-1 (Apaf-1) leads to the formation of the apoptosome and activation of a cascade of caspases, highlighting the critical role of cytochrome c in controlling cell death mechanisms. Additionally, cytochrome c undergoes post-translational modifications, especially phosphorylation, which intricately regulate its roles in both respiration and apoptosis. These modifications add layers of complexity to how cytochrome c effectively controls cellular functions. cytochrome c becomes a lighthouse in the intricate web of cancer, its expression patterns providing hints about prognosis and paths toward treatment. Reduced levels of cytochrome c have been observed in cancer tissues, indicating a potential inhibition of apoptosis. For instance, in glioma tissues, cytochrome c levels were lower compared to healthy tissues, and this reduction became more pronounced in advanced stages of the disease. However, the role of cytochrome c in cancer becomes more intricate as it becomes intertwined with the metabolic reprogramming of cancer cells. This suggests that cytochrome c plays a crucial role in tumor progression and resistance to treatment. Viewing cytochrome c as a molecular mosaic reveals that it is not merely a protein, but also a central player in determining cellular fate. This realization opens up exciting avenues for potential advancements in cancer diagnosis and treatment strategies. Despite the advancements made, the narrative surrounding cytochrome c remains incomplete, urging further exploration into its complexities and the biological implications linked to cancer. cytochrome c stands as a beacon of hope and a promising target for therapy in the battle against cancer, particularly due to its significant involvement in tumor metabolism. It holds the potential for a future where innovative solutions can be developed to address the intricate challenges of cellular fate. In this review, we have endeavored to illuminate the multifaceted domain of cytochrome c drawing connections among apoptosis, metabolic reprogramming, and the Warburg effect in the context of cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Saudi Pharmaceutical Journal
PHARMACOLOGY & PHARMACY-
CiteScore
6.10
自引率
2.40%
发文量
194
审稿时长
67 days
期刊介绍:
The Saudi Pharmaceutical Journal (SPJ) is the official journal of the Saudi Pharmaceutical Society (SPS) publishing high quality clinically oriented submissions which encompass the various disciplines of pharmaceutical sciences and related subjects. SPJ publishes 8 issues per year by the Saudi Pharmaceutical Society, with the cooperation of the College of Pharmacy, King Saud University.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


