两个关键的解毒酶基因 NlCYP301B1 和 NlGSTm2 赋予褐飞虱 Nilaparvata lugens Stål 抗百草枯性
IF 4.2
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
褐飞虱 Nilaparvata lugens Stål 是一种臭名昭著的害虫,为害亚洲各地的水稻。褐飞虱对化学杀虫剂抗药性的快速演变对农业和人类健康构成了持续威胁。目前,吡虫啉已成为治理 N. lugens 的一种可行的吡虫啉替代品。昆虫对杀虫剂的解毒包括三大代谢基因家族:细胞色素 P450 单氧化酶(P450s)、谷胱甘肽 S 转移酶(GSTs)和羧基酯酶(CarEs)。在这项研究中,通过连续多重选择,从易感良性前列腺增生菌株中培育出了对吡美曲嗪高度耐药的菌株(BPH-R40:705倍)。测定了解毒酶(包括 P450s、GSTs 和 CarEs)的活性。值得注意的是,抗吡咯嗪菌株的 P450s 和 GSTs 活性明显高于易感 BPH 菌株。因此,我们对 N. lugens 的 P450s 和 GSTs 基因进行了鉴定,并揭示了它们的系统发育、结构、基序分析和染色体位置。随后,我们对 53 个 P450s 基因和 11 个 GSTs 基因的表达谱进行了定量分析,并确定了 NlCYP301B1 和 NlGSTm2 这两个关键的解毒酶基因参与了百菌清抗性的形成。此外,以 RNA 干扰(RNAi)为介导的 NlCYP301B1 和 NlGSTm2 基因表达沉默能显著提高 BPH 幼虫对吡蚜酮的死亡率。据我们所知,提高关键解毒酶的活性以抵御杀虫剂是昆虫中一种广泛而重要的防御机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
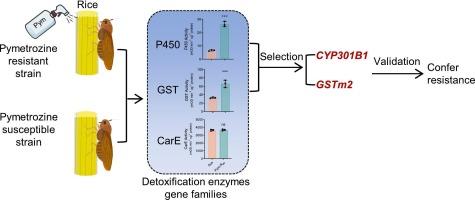
Two critical detoxification enzyme genes, NlCYP301B1 and NlGSTm2 confer pymetrozine resistance in the brown planthopper (BPH), Nilaparvata lugens Stål
The brown planthopper (BPH), Nilaparvata lugens Stål, is a notorious pest that infests rice across Asia. The rapid evolution of chemical pesticide resistance in BPH poses an ongoing threat to agriculture and human health. Currently, pymetrozine has emerged as a viable alternative to imidacloprid for managing N. lugens. The detoxification of insecticides in insects includes three major metabolic gene families: cytochrome P450 monooxygenases (P450s), glutathione S-transferases (GSTs), and carboxylesterases (CarEs). In this study, highly resistant strains of BPH to pymetrozine (BPH-R40: 705-fold) were created from the susceptible BPH strain through continuous multi-selection. The activities of detoxifying enzymes, including P450s, GSTs, and CarEs were measured. Notably, P450s and GSTs exhibited significantly higher activity in the pymetrozine-resistance strain than that of the susceptible BPH strain. Hence, we characterized P450s and GSTs genes in N. lugens and revealed their phylogeny, structure, motif analysis, and chromosome location. Subsequently, the expression profiles of 53 P450s and 11 GSTs genes were quantified, and two crucial detoxifying enzyme genes, NlCYP301B1 and NlGSTm2, were identified as being involved in pymetrozine resistance. Furthermore, RNA interference (RNAi)-mediated silencing of NlCYP301B1 and NlGSTm2 gene expression significantly increased larval mortality of BPH in response to pymetrozine. To our knowledge, enhancing the activity of key detoxification enzymes to resist insecticides represents a widespread and vital defense mechanism in insects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


