工程纳米平台介导的气体疗法增强了体内治疗肿瘤的铁氧化作用
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
高谷胱甘肽(GSH)环境对诱导肿瘤细胞铁突变构成了巨大挑战,因此有必要开发可消耗细胞内GSH的纳米平台。在这项研究中,我们开发了一种工程纳米平台(MIL-100@Era/L-Arg-HA),它能通过气体疗法增强铁突变。首先,我们证实了该纳米平台中的铁元素在肿瘤细胞的高GSH和H2O2影响下发生价态变化。同时,L-Arg 在细胞内 H2O2 的存在下生成 NO 气体,并与 GSH 发生反应。此外,Erastin 还通过抑制胱氨酸/谷氨酸抗转运体系统、减少胱氨酸摄取和损害 GPX4 来消耗 GSH,同时还通过激活 NOX4 蛋白表达来增加细胞内 H2O2 水平。通过这些联合的 GSH 消耗机制,我们证明 MIL-100@Era/L-Arg-HA 在体外有效地消耗了 GSH 水平,破坏了 GPX4 的功能,并增加了细胞内脂质 ROS 水平。此外,这种纳米平台还能明显抑制肿瘤细胞的生长,延长肿瘤小鼠的体内存活时间。这种通过气体疗法增强铁突变的工程纳米平台为基于铁突变的癌症疗法带来了巨大前景,并为临床肿瘤治疗提供了潜在策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
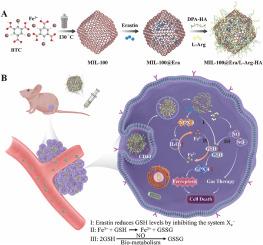
Engineered nanoplatform mediated gas therapy enhanced ferroptosis for tumor therapy in vivo
The high glutathione (GSH) environment poses a significant challenge for inducing ferroptosis in tumor cells, necessitating the development of nanoplatforms that can deplete intracellular GSH. In this study, we developed an engineered nanoplatform (MIL-100@Era/L-Arg-HA) that enhances ferroptosis through gas therapy. First, we confirmed that the Fe element in the nanoplatform undergoes valence changes under the influence of high GSH and H2O2 in tumor cells. Meanwhile, L-Arg generates NO gas in the presence of intracellular H2O2, which reacts with GSH. Additionally, Erastin depletes GSH by inhibiting the cystine/glutamate antiporter system, reducing cystine uptake and impairing GPX4, while also increasing intracellular H2O2 levels by activating NOX4 protein expression. Through these combined GSH-depletion mechanisms, we demonstrated that MIL-100@Era/L-Arg-HA effectively depletes GSH levels, disrupts GPX4 function, and increases intracellular lipid ROS levels in vitro. Furthermore, this nanoplatform significantly inhibited tumor cell growth and extended the survival time of tumor-bearing mice in vivo. This engineered nanoplatform, which enhances ferroptosis through gas therapy, shows significant promise for ferroptosis-based cancer therapy and offers potential strategies for clinical tumor treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


