纳米粘土凝胶可减轻 BMP2 相关炎症并促进软骨生成,从而增强 BMP2-脊髓融合能力
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
骨形态发生蛋白 2(BMP2)具有成骨作用,因此被临床用于治疗难治性骨折和促进脊柱融合。然而,胶原蛋白载体释放超生理剂量的 BMP2 后产生不良反应的报道屡见不鲜。纳米粘土凝胶(NC)作为一种生物材料备受关注,因为它有可能使施用的药剂产生局部疗效。然而,NC/BMP2 的功效和作用机制仍不清楚。本研究探讨了 NC 作为 BMP2 载体在骨再生中的功效及其增强机制。与海绵胶原(CS)相比,筋膜下植入含有BMP2的NC能促进骨形成。软骨在 NC 内部均匀形成,而 CS 仅在周边形成软骨。此外,CS 会在植入部位周围诱发剂量依赖性炎症反应,而 NC 只诱发轻微反应,并且在 NC 内部也能观察到炎症细胞。在大鼠脊柱融合模型中,与CS相比,NC能促进高质量的骨融合。在体外,NC增强了hBMSCs和ATDC5细胞的软骨和成骨分化,同时抑制了破骨细胞的生成。总之,NC/BMP2 可促进空间可控的高质量软骨内骨形成,且不会引起 BMP2 诱导的炎症,还能促进高密度新骨的形成,可作为新一代 BMP2 载体发挥作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
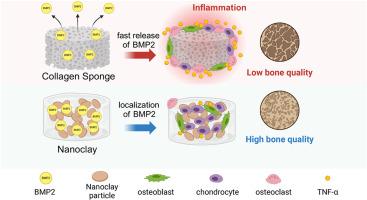
Nanoclay gels attenuate BMP2-associated inflammation and promote chondrogenesis to enhance BMP2-spinal fusion
Bone morphogenetic protein 2 (BMP2) is clinically applied for treating intractable fractures and promoting spinal fusion because of its osteogenic potency. However, adverse effects following the release of supraphysiological doses of BMP2 from collagen carriers are widely reported. Nanoclay gel (NC) is attracting attention as a biomaterial, given the potential for localized efficacy of administered agents. However, the efficacy and mechanism of action of NC/BMP2 remain unclear. This study explored the efficacy of NC as a BMP2 carrier in bone regeneration and the enhancement mechanism. Subfascial implantation of NC containing BMP2 elicited superior bone formation compared with collagen sponge (CS). Cartilage was uniformly formed inside the NC, whereas CS formed cartilage only on the perimeter. Additionally, CS induced a dose-dependent inflammatory response around the implantation site, whereas NC induced a minor response, and inflammatory cells were observed inside the NC. In a rat spinal fusion model, NC promoted high-quality bony fusion compared to CS. In vitro, NC enhanced chondrogenic and osteogenic differentiation of hBMSCs and ATDC5 cells while inhibiting osteoclastogenesis. Overall, NC/BMP2 facilitates spatially controlled, high-quality endochondral bone formation without BMP2-induced inflammation and promotes high-density new bone, functioning as a next-generation BMP2 carrier.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


