用于预防艾滋病、HSV-2 和意外怀孕的新一代 3D 打印多用途阴道内避孕环。
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在全球范围内,近一半的妊娠是意外怀孕,每年新增约 130 万例人类免疫缺陷病毒(HIV)感染病例,估计有超过 5 亿人感染生殖器单纯疱疹病毒(HSV-2)。在此,我们报告了首个用于预防 HIV、HSV-2 和意外怀孕的 3D 打印多用途预防技术(MPT)阴道内环(IVR)。阴道内避孕环采用最先进的连续液体界面生产(CLIP™)3D 打印技术,使用生物相容性硅酮-聚氨酯树脂制成。抗艾滋病毒药物(达匹韦林,DPV)、抗疱疹药物(普利特韦,PTV)和避孕药物(左炔诺孕酮,LNG)被装载在猕猴大小的 IVR 中(外径 25 毫米,OD;横截面 6.0 毫米,CS),与人类大小(外径 54 毫米;横截面 7.6 毫米)的 IVR 类似物成比例。所有三种活性药物成分(API)都通过吸收驱动的单步药物加载过程加载到 IVR 中。在体外模拟阴道液(SVF)中,DPV、PTV 和 LNG 分别在与人和猕猴阴道 pH 值相关的 pH 4 和 pH 8 条件下产生零阶释放动力学。CLIP 三维打印的 MPT IVR 在 4 °C 下储存 6 个月后仍保持稳定,物理、尺寸或机械性能没有变化,药物浓度没有变化,也没有药物降解副产物。MPT IVR 在猕猴体内持续释放三种原料药达 28 天,血浆中位浓度分别为 138 pg/mL(DPV)、18,700 pg/mL(PTV)和 335 pg/mL(LNG)。安全性研究表明,MPT IVR 对猕猴安全且耐受性良好,阴道 pH 值未观察到变化或异常,测试的 22 种粘膜细胞因子和趋化因子中,包括促炎细胞因子(IL-1β、IL-6、IL-8、IFN-γ、TNF-α、IL-17、IL-18)和抗炎细胞因子(IL-10、IL-12)在放置 MPT IVR 时或移除后均无显着变化。此外,在整个研究过程中,MPT IVR 不会引起全身 CD4+ 和 CD8+ T 细胞的变化。总之,拟议的 MPT IVR 有可能为年轻妇女和女孩提供更多预防意外怀孕和两种高发性传播感染 (STI) 的选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
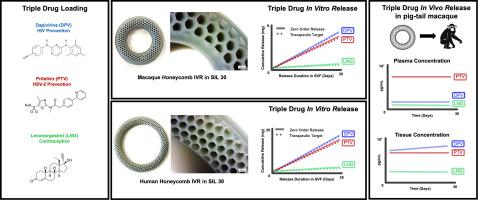
Next-generation 3D printed multipurpose prevention intravaginal ring for prevention of HIV, HSV-2, and unintended pregnancy
Globally, nearly half of all pregnancies are unintended, ∼1.3 million new human immunodeficiency virus (HIV) infections are reported every year, and more than 500 million people are estimated to have a genital herpes simplex virus (HSV-2) infection. Here we report the first 3D printed multipurpose prevention technology (MPT) intravaginal ring (IVR) for prevention of HIV, HSV-2, and unintended pregnancy. The IVRs were fabricated using state-of-the-art Continuous Liquid Interface Production (CLIP™) 3D printing technology using a biocompatible silicone-urethane based resin. Anti-HIV drug (Dapivirine, DPV), anti-herpes drug (Pritelivir, PTV) and a contraceptive drug (Levonorgestrel, LNG) were loaded in a macaque size IVR (25 mm outer diameter, OD; 6.0 mm cross-section, CS) allometrically scaled from the human size (54 mm OD; 7.6 mm CS) IVR analogue. All three active pharmaceutical ingredients (APIs) were loaded in the IVR using a single-step drug loading process driven by absorption. DPV, PTV, and LNG elicited zero-order release kinetics in vitro in simulated vaginal fluid (SVF) at pH 4 and pH 8 relevant to human and macaque vaginal pH respectively. CLIP 3D printed MPT IVRs remained stable after 6 months of storage at 4 °C with no change in physical, dimensional, or mechanical properties and no change in drug concentration and absence of drug degradation byproducts. The MPT IVRs elicited sustained release of all three APIs in macaques for 28 days with median plasma concentrations of 138 pg/mL (DPV), 18,700 pg/mL (PTV), and 335 pg/mL (LNG). Safety studies demonstrated that the MPT IVRs were safe and well tolerated in the macaques with no observed change or abnormalities in vaginal pH and no significant changes in any of the 22 mucosal cytokines and chemokines tested including pro-inflammatory (IL-1β, IL-6, IL-8, IFN-γ, TNF-α, IL-17, IL-18) and anti-inflammatory (IL-10, IL-12) cytokines while the MPT IVR was in place or after its removal. Additionally, MPT IVRs elicited no observed alterations in systemic CD4+ and CD8+ T cells during the entire study. Collectively, the proposed MPT IVR has potential to expand preventative choices for young women and girls against unintended pregnancy and two highly prevalent sexually transmitted infections (STIs).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


