甲基丙烯酸明胶基多孔微球和多细胞装配的协同作用,促进骨组织重建中的成骨和血管生成。
IF 7.7
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2024-11-03
DOI:10.1016/j.ijbiomac.2024.137228
引用次数: 0
摘要
骨缺损治疗的关键挑战之一是在新组织再生过程中提供充足而稳定的血液供应。间充质干细胞(MSCs)和内皮细胞(ECs)通过旁分泌效应在骨缺损修复过程中促进成骨和血管生成的潜力巨大,但其治疗效果取决于有效的细胞组装和递送。在这项工作中,我们结合微流控技术和梯度冷冻干燥技术,开发了多种不同孔径的微球,用于多细胞递送,以增强血管生成和成骨能力。通过调节水凝胶微球的冷冻时间,可以控制制备的多孔甲基丙烯酸明胶(GelMA)基水凝胶微球(PGMS)的颗粒和孔径,颗粒和孔径范围分别为150-250 μm和10-100 μm,冷冻时间为0 min至30 min。研究发现,优化后的颗粒大小(200.8 ± 14.2 μm)和孔径大小(11.2 ± 1.9 μm)可促进细胞在 PGMS 中的组装、粘附、生长和增殖。此外,还实现了骨髓间充质干细胞(BMSCs)和人脐静脉内皮细胞(HUVECs)在 PGMS 上的共同组装和输送,并确定了 BMSCs 和 HUVECs 共同培养的最佳细胞比例(20:2),以实现最佳的旁分泌效应,进一步促进成骨分化和血管生成。最后,体外和体内实验结果表明,所开发的 BMSCs 与 HUVECs 共同组装的 PGMS 有助于加速骨再生和血管化过程,在血管化骨组织重建中展现出巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
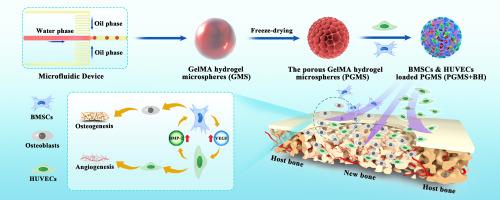
Synergy of engineered gelatin methacrylate-based porous microspheres and multicellular assembly to promote osteogenesis and angiogenesis in bone tissue reconstruction
One of the key challenges in bone defects treatment is providing adequate and stable blood supply during new tissue regeneration. Mesenchymal stem cells (MSCs) and endothelial cells (ECs) have great potential to promote osteogenesis and angiogenesis during bone defect repair through paracrine effects, but their therapeutic efficacy depends on effective cellular assembly and delivery. In this work, we developed various microspheres with different pore sizes for multi-cellular delivery to enhance the angiogenic and osteogenic capability via combining microfluidic and gradient freeze-drying techniques. The particle and pore size of fabricated porous gelatin methacrylate (GelMA)-based hydrogel microspheres (PGMS) could be controllable through adjusting the freezing time of hydrogel microspheres, the range of particles and pores size are 150–250 μm and 10–100 μm with different freezing time from 0 min to 30 min. The optimized particle size (200.8 ± 14.2 μm) and pore size (11.2 ± 1.9 μm) were explored to promote cell assemble, adhesion, growth, and proliferation in the PGMS. Furthermore, the co-assembly and delivery of bone marrow mesenchymal stem cells (BMSCs) and human umbilical vein endothelial cells (HUVECs) on the PGMS was achieved and an optimal cellular ratio of BMSCs to HUVECs (20:2) was established for co-culturing of them to achieve optimal paracrine effects, further promoting osteogenic differentiation and angiogenesis. Finally, results from both in vitro and in vivo experiments showed that the developed PGMS with co-assembly of BMSCs to HUVECs contributed to accelerate bone regeneration and vascularization process daringly, exhibited great potential in vascularized bone tissue reconstruction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


