分子动力学模拟中混合的显式构型熵。
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
The Journal of Physical Chemistry Letters
Pub Date : 2024-11-14
Epub Date: 2024-11-05
DOI:10.1021/acs.jpclett.4c02819
引用次数: 0
摘要
多组分粒子系统的混合熵是平衡态摩尔分数的简单表达式,但其瞬态(非平衡态)的中间值至今无法直接从粒子坐标中计算出来。我们提出了一种仅基于瞬时坐标集的简单混合构型熵表达式,适用于沿分子动力学轨迹即时确定混合程度。我们以分子动力学模拟中表现出快速和慢速混合和脱混合过程的几种分子混合物为例,说明了我们方案的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
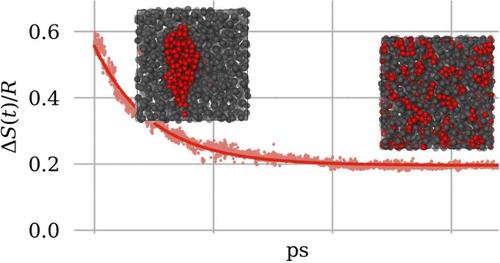
Explicit Configurational Entropy of Mixing in Molecular Dynamics Simulations.
The entropy of mixing of a multicomponent system of particles is a simple expression of the molar fractions for the equilibrium state, but its intermediate values for transient (nonequilibrium) states can not be calculated directly from the particle coordinates so far. We propose a simple expression for the configurational entropy of mixing based solely on the set of instantaneous coordinates, which is suitable for the on-the-fly determination of the degree of mixing along a molecular dynamics trajectory. We illustrate the applicability of our scheme with the example of several molecular mixtures that exhibit fast and slow mixing and demixing processes within a molecular dynamics simulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


