在可见光辐照下,通过金(I)催化邻烷基苯甲酸的炔基环化作用生成 3-炔基苯酞。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-15
Epub Date: 2024-11-06
DOI:10.1021/acs.joc.4c01876
引用次数: 0
摘要
在此,我们报告了一个 Csp2-Csp 交叉偶联过程,该过程涉及金催化与可见光光催化的合并,导致邻炔基苯甲酸的炔基环化。以 E:Z 异构体混合物的形式获得了相应的、以前未曾描述过的亚烷基酞产品。关键的 C-C 键的形成是基于炔基碘化物与乙烯基金(I)中间体的氧化加成的光活化,而乙烯基金(I)中间体是由最初的 5-exo-dig 环化途径产生的,这一点得到了包括 DFT 计算在内的机理研究的支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
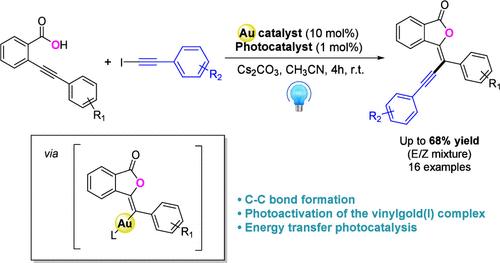
Formation of 3-Alkynylidenephthalides by Gold(I)-Catalyzed Alkynylative Cyclization of o-Alkynylbenzoic Acids under Visible Light Irradiation.
Herein, we report a Csp2-Csp cross-coupling process involving the merger of gold catalysis and visible light photocatalysis leading to the alkynylative cyclization of o-alkynyl benzoic acids. The corresponding and previously undescribed alkynylidenephthalide products were obtained as mixtures of E:Z isomers. The key C-C bond formation is based on the photoactivation of the oxidative addition of an alkynyliodide to a vinylgold(I) intermediate resulting from an initial 5-exo-dig cyclization pathway, as supported by mechanistic studies including DFT calculations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


