通过与 N-卤代丁二酰亚胺反应制备 2-亚甲基氧杂环丁烷:反应物对立体化学结果和反应途径的影响。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-15
Epub Date: 2024-11-05
DOI:10.1021/acs.joc.4c01877
引用次数: 0
摘要
本研究首次报道了通过 2-亚甲基氧杂环丁烷与 N-卤代丁二酰亚胺(NXS)反应合成 2-亚甲基氧杂环丁烷的一般方法。转化的相对非对映选择性取决于 NXS 的卤素,而其他反应结果则受到氧杂环丁烷上取代基的影响。本文介绍了探索所观察到的非对映选择性基础的量子力学计算和分子动力学模拟。这些高度紧张的杂环进行了标准的交叉耦合反应,证明了它们作为合成中间体的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
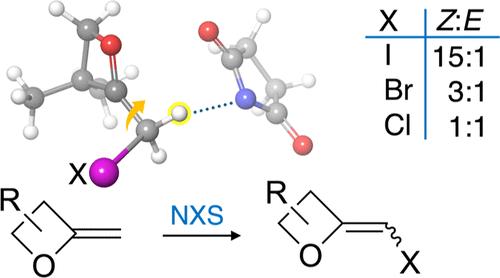
2-Halomethyleneoxetanes from 2-Methyleneoxetanes by Reaction with N-Halosuccinimides: Reactant Influences on Stereochemical Outcomes and Reaction Pathways.
The first general synthesis of 2-halomethyleneoxetanes, realized by the reaction of 2-methyleneoxetanes with N-halosuccinimides (NXS), is reported. The relative diastereoselectivities of the transformations were dependent on the halogen of NXS, while alternative reaction outcomes were influenced by substituents on the oxetane. Quantum mechanical calculations and molecular dynamics simulations exploring the basis of the observed diastereoselectivities are described. These highly strained heterocycles underwent standard cross-coupling reactions, demonstrating their utility as synthetic intermediates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


