SOP-MULTI:基于自组织聚合物的多域和本征紊乱蛋白质粗粒度模型,其构象组合与实验散射数据一致
IF 5.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
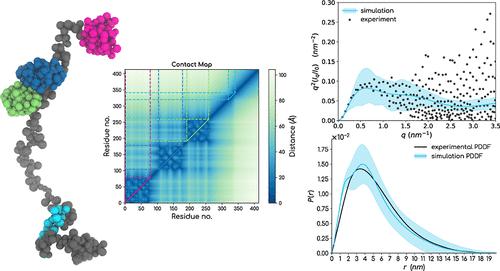
具有长柔性连接体的多域蛋白质和全长内在无序蛋白质(IDPs)最好被定义为构象的集合而非单一结构。利用结构生物物理学实验工具确定这类蛋白质的高分辨率集合结构面临着各种挑战。目前,将可用的低分辨率集合平均实验数据与硅学生物分子重构相结合的综合方法经常被用于这一目的。然而,对大型蛋白质进行广泛的玻尔兹曼加权构象采样,尤其是对折叠结构域和无序结构域都存在于同一多肽链中的蛋白质进行采样,仍然是一项挑战。在这项工作中,我们提出了一种每个氨基酸分辨率为 2 位点的 SOP-MULTI 力场,用于模拟多结构域蛋白质的粗粒度模型。SOP-MULTI 结合了两种成熟的自组织聚合物模型:(i) 用于折叠系统的 SOP-SC 模型和 (ii) 用于 IDPs 的 SOP-IDP 模型。对于 SOP-MULTI,我们在属于折叠区和无序区的珠子之间引入了交叉相互作用项,以生成全长多域蛋白质的构象组合,如 hnRNP A1、TDP-43、G3BP1、hGHR-ECD、TIA1、HIV-1 Gag、多泛素和 FUS。当反向映射到全原子分辨率时,SOP-MULTI轨迹忠实地再现了倒数空间范围内的散射数据。我们还表明,相对于已解决的折叠结构,单个折叠结构域保留了原生接触,而且折叠结构域中残基的均方根波动与相同折叠系统的全原子分子动力学模拟轨迹相吻合。SOP-MULTI 力场是一个与 LAMMPS 兼容的用户软件包,并附有设置代码,用于生成任何具有折叠和无序区域的全长蛋白质所需的文件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
SOP-MULTI: A Self-Organized Polymer-Based Coarse-Grained Model for Multidomain and Intrinsically Disordered Proteins with Conformation Ensemble Consistent with Experimental Scattering Data
Multidomain proteins with long flexible linkers and full-length intrinsically disordered proteins (IDPs) are best defined as an ensemble of conformations rather than a single structure. Determining high-resolution ensemble structures of such proteins poses various challenges by using tools from experimental structural biophysics. Integrative approaches combining available low-resolution ensemble-averaged experimental data and in silico biomolecular reconstructions are now often used for the purpose. However, extensive Boltzmann weighted conformation sampling for large proteins, especially for ones where both the folded and disordered domains exist in the same polypeptide chain, remains a challenge. In this work, we present a 2-site per amino-acid resolution SOP-MULTI force field for simulating coarse-grained models of multidomain proteins. SOP-MULTI combines two well-established self-organized polymer models─: (i) SOP-SC models for folded systems and (ii) SOP-IDP for IDPs. For the SOP-MULTI, we introduce cross-interaction terms between the beads belonging to the folded and disordered regions to generate conformation ensembles for full-length multidomain proteins such as hnRNP A1, TDP-43, G3BP1, hGHR-ECD, TIA1, HIV-1 Gag, polyubiquitin, and FUS. When back-mapped to all-atom resolution, SOP-MULTI trajectories faithfully recapitulate the scattering data over the range of the reciprocal space. We also show that individual folded domains preserve native contacts with respect to solved folded structures, and root-mean-square fluctuations of residues in folded domains match those obtained from all-atom molecular dynamics simulation trajectories of the same folded systems. SOP-MULTI force field is made available as a LAMMPS-compatible user package along with setup codes for generating the required files for any full-length protein with folded and disordered regions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical Theory and Computation
化学-物理:原子、分子和化学物理
CiteScore
9.90
自引率
16.40%
发文量
568
审稿时长
1 months
期刊介绍:
The Journal of Chemical Theory and Computation invites new and original contributions with the understanding that, if accepted, they will not be published elsewhere. Papers reporting new theories, methodology, and/or important applications in quantum electronic structure, molecular dynamics, and statistical mechanics are appropriate for submission to this Journal. Specific topics include advances in or applications of ab initio quantum mechanics, density functional theory, design and properties of new materials, surface science, Monte Carlo simulations, solvation models, QM/MM calculations, biomolecular structure prediction, and molecular dynamics in the broadest sense including gas-phase dynamics, ab initio dynamics, biomolecular dynamics, and protein folding. The Journal does not consider papers that are straightforward applications of known methods including DFT and molecular dynamics. The Journal favors submissions that include advances in theory or methodology with applications to compelling problems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


