水热老化对 CeWOx 上标准和快速 SCR 的影响:关于吸附位点和反应机理的定量研究
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
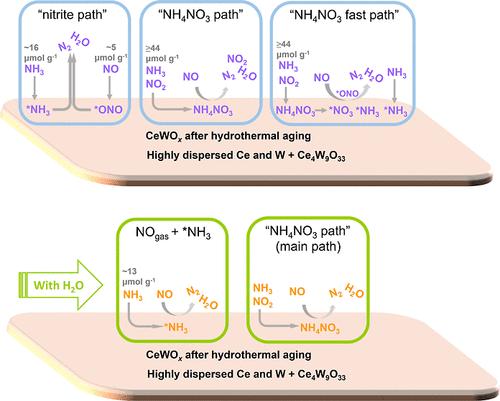
CeWOx 在 700 ℃ 水热老化 10 小时后,在 200-450 ℃ 快速选择性催化还原氮氧化物(快速 SCR)过程中,无论有无 H2O 存在,均可实现 90% 的氮氧化物(NOx)转化率,高于或媲美文献报道的其他未经水热老化的氧化物催化剂。即使在 800 °C 水热老化 10 小时后,CeWOx 仍可在 200-400 °C 下实现 80% 的氮氧化物转化率,无论是否存在 H2O。水热老化后,老化 CeWOx 对 NO 和 NH3 的吸附量急剧下降,NO 活化、硝酸盐生成和 "亚硝酸盐路径"(Langmuir-Hinshelwood 机制)受到抑制,导致标准 SCR 过程中 NOx 转化率急剧下降。在快速选择性催化还原过程中,虽然 NO 的吸附量较低,但气态 NO2 和 NH3 相互作用形成的 NH4NO3 增加了 NO2 和 NH3 的吸附量,弥补了吸附位点的损失。NH4NO3 参与了 "NH4NO3 快速路径 "和 "NH4NO3 路径"(Eley-Rideal 机理),是获得高活性的重要中间产物。这些都是快速 SCR 保持优异活性的原因。水的存在抑制了 "亚硝酸盐路径 "和 "NH4NO3 路径",导致氮氧化物转化率降低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effects of Hydrothermal Aging on Standard and Fast SCR over CeWOx: A Quantitative Study on Adsorption Sites and Reaction Mechanism
CeWOx after hydrothermal aging at 700 °C for 10 h exhibited >90% nitrogen oxide (NOx) conversion at 200–450 °C either in the presence or absence of H2O during fast selective catalytic reduction of NOx (fast SCR), which was higher than or comparable to other oxide catalysts without hydrothermal aging reported in the literature. Even after hydrothermal aging at 800 °C for 10 h, CeWOx could achieve >80% NOx conversion at 200–400 °C, either in the presence or absence of H2O. After hydrothermal aging, the adsorption amounts of NO and NH3 on aged CeWOx decreased dramatically, and NO activation, nitrate production, and the “nitrite path” (Langmuir–Hinshelwood mechanism) were inhibited, causing a drastic decline in NOx conversion during standard SCR. During fast SCR, although the adsorption amount of NO was low, the interaction between gaseous NO2 and NH3 to form NH4NO3 increased the adsorption amounts of NO2 and NH3, compensating for the loss of adsorption sites. NH4NO3 took part in the “NH4NO3 fast path” and “NH4NO3 path” (Eley–Rideal mechanism) and was an important intermediate for obtaining a high activity. These are the reasons why fast SCR maintains an excellent activity. The presence of water inhibited the “nitrite path” and “NH4NO3 path”, leading to decreased NOx conversion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


