手性 2-氨基和 1-苯基-2-氨基二烯的合成及其在双烯-阿尔德反应中的应用,以获得手性环酮
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究报道了利用钯催化从一系列手性噁唑烷酮制备手性 2-氨基和 2-氨基-1-苯基-1,3-二烯的方法。这种钯催化的碳氮键形成反应可提供相应的手性氨基二烯,收率从中等到极好(12 例,高达 97%)。在 Diels-Alder (DA) 反应中,生成的手性氨基二烯被用作新型二烯(58 个实例,高达 93:7 dr,收率高达 70%)。水解后可得到一系列手性环酮,产物具有很高的对映选择性(ee高达 92%,收率高达 81%)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
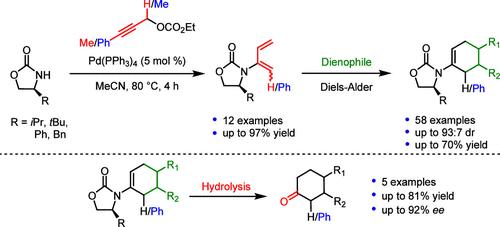
Synthesis and Application of Chiral 2-Amido and 1-Phenyl-2-amido Dienes in Diels–Alder Reactions to Access Chiral Cyclic Ketones
The preparation of a focused library of chiral 2-amido and 2-amido-1-phenyl-1,3-dienes from a range of chiral oxazolidinones using palladium-catalysis is reported. This palladium-catalyzed carbon–nitrogen bond-forming reaction provides the corresponding chiral amido-dienes in moderate to excellent yields (12 examples, up to 97%). The resulting chiral amido-dienes are employed as novel dienes in Diels–Alder (DA) reactions (58 examples, up to 93:7 dr, up to 70% yield). A range of chiral cyclic ketones were accessed upon hydrolysis, affording products with high levels of enantioselectivity (up to 92% ee, up to 81% yield).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


