纳米黏土介导的感染伤口愈合双管齐下策略
IF 9.6
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
光热疗法(PTT)是对抗细菌感染和规避多药耐药性的有效方法。然而,如何在有效杀灭细菌和减少对周围正常组织损伤之间取得平衡仍是一个巨大的挑战。本文构建了一种高度协同的普鲁士蓝/高岭石(PB/Kaol)杂交纳米系统,用于抗菌治疗,加速感染伤口的愈合。与 Kaol 杂交后,制备的 PB/Kaol 形成了界面 Al-O-Fe 键,这是 Kaol 向 PB 的快速电荷转移通道,有助于增强 PB/Kaol 的光热效应。此外,Kaol 表面的羟基和路易斯酸碱位点可促进 PB/Kaol 与细菌的粘附,从而确保尽可能多的热量集中在细菌上,最大限度地减少对周围健康组织的损害。此外,PB/Kaol 还继承了 PB 和 Kaol 的抗炎和止血功能,使感染伤口迅速愈合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
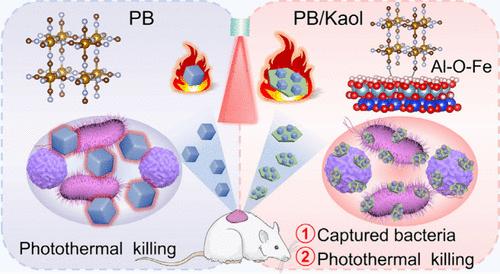
Nanoclay Mediated Two-Pronged Strategy for Infected-Wound Healing
Photothermal therapy (PTT) is an efficient way to combat bacterial infections and circumvent multidrug resistance. However, balancing efficacious bacterial killing and minimizing damage to the surrounding normal tissues remain a great challenge. Herein, a highly cooperative Prussian blue/kaolinite (PB/Kaol) hybrid nanosystem is constructed for antibacterial therapy to accelerate the healing of infected wounds. After hybridization with Kaol, the prepared PB/Kaol forms interfacial Al–O–Fe bonds, a fast charge transfer channel from Kaol to PB, which contributes to the enhanced photothermal effect of PB/Kaol. Additionally, the hydroxyl and Lewis acid–base sites of the Kaol surface could promote the adhesion of PB/Kaol to bacteria, thereby ensuring that as much hyperthermia as possible is focused on the bacteria and minimizing damage to the surrounding healthy tissues. Furthermore, PB/Kaol inherits the anti-inflammatory and hemostasis functions of PB and Kaol, resulting in the rapid healing of infected wounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


