揭示用于高性能锂石榴石固态电池的超快烧结 LLZO 固态电解质的表面化学性质
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
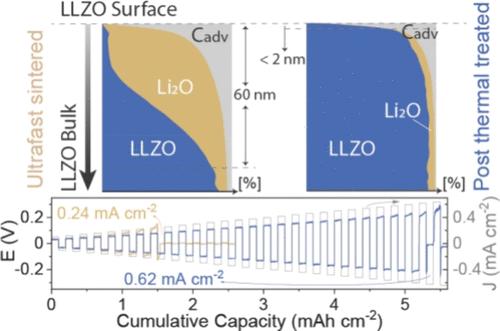
超快(UF)烧结是制造 Li7La3Zr2O12(LLZO)固态电解质的一种改变游戏规则的烧结方法,代表着向锂石榴石固态电池的进步和未来商业化迈出的关键一步。尽管超滤被广泛应用于 LLZO 陶瓷的制造,但超滤烧结 LLZO 表面的化学成分在很大程度上仍未得到研究。本研究采用综合技术,包括深度探测 X 射线光电子能谱 (XPS) 和聚焦离子束飞行时间二次离子质谱 (FIB-TOF-SIMS),对超滤烧结 LLZO 的表面化学成分进行了深入分析。我们的研究揭示了超滤烧结与传统烧结 LLZO 表面之间的显著差异,发现在超滤处理的情况下,Li2O 的主要表面污染深度可达约 40 nm。超滤烧结和传统烧结过程中的同步辐射 X 射线衍射数据对比阐明了表面污染的来源。通过 XPS 和 FIB-TOF-SIMS 测量证实,我们提出了一个可行的解决方案,即在超滤烧结后在 900 ℃ 下进行额外的热处理 (HT)。此外,我们还对基于超滤烧结 LLZO 颗粒的锂/LLZO/锂对称电池的电化学性能进行了比较评估,包括采用后热处理步骤和未采用后热处理步骤,从而强调了未受污染的 LLZO 表面的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unveiling Surface Chemistry of Ultrafast-Sintered LLZO Solid-State Electrolytes for High-Performance Li-Garnet Solid-State Batteries
Ultrafast (UF) sintering emerges as a game-changing sintering methodology for fabricating Li7La3Zr2O12 (LLZO) solid-state electrolytes, representing a pivotal stride toward the advancement and prospective commercialization of Li-garnet solid-state batteries. Despite its widespread use in the fabrication of LLZO ceramics, the chemical composition of the UF-sintered LLZO surface remains largely unexplored. This study presents an in-depth analysis of the surface chemistry of UF-sintered LLZO using comprehensive techniques, including depth-profiling X-ray photoelectron spectroscopy (XPS) and focused-ion-beam time-of-flight secondary ion mass spectroscopy (FIB-TOF-SIMS). Our investigation uncovers a striking difference between the surface of UF-sintered and conventionally sintered LLZO, revealing predominant surface contamination by Li2O up to ca. 40 nm depth in the case of UF processing. Comparative synchrotron X-ray diffraction data during UF and conventional sintering elucidate the origin of surface contamination. We propose a viable solution to this issue through an additional heat treatment (HT) step at 900 °C after UF sintering, as corroborated by XPS and FIB-TOF-SIMS measurements. Furthermore, we present a comparative assessment of the electrochemical performance of Li/LLZO/Li symmetric cells based on UF-sintered LLZO pellets, both with and without the post-HT step, underscoring the pivotal role of an uncontaminated LLZO surface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


