多模式靶向嵌合体可利用肿瘤免疫微环境实现综合免疫疗法
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
虽然免疫疗法给癌症治疗带来了革命性的变化,但其疗效受到多种因素的影响,尤其是肿瘤免疫微环境(TIME)的复杂性和异质性。在 TIME 中同时协同调动多种免疫细胞的策略仍然非常理想,但却极具挑战性。在此,我们报告了一种多模式和可编程平台,它能将多种治疗模块整合到单一药物中,以肿瘤为靶点共同参与 TIME 中的多种免疫细胞。我们开发了三重正交链接器(T-Linker)技术,将各种治疗小分子和生物大分子整合为多模态靶向嵌合体(Multi-TACs)。表皮生长因子受体-CD3-PDL1多TAC促进了T-树突状细胞共同参与靶向实体瘤,在体外、几种人源化小鼠模型和患者衍生肿瘤模型中均表现出卓越的疗效。此外,Multi-TAC 还能协调 T 细胞与其他免疫细胞类型。我们的 Multi-TACs 具有高度模块化和可编程的特点,可广泛应用于免疫疗法及其他领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
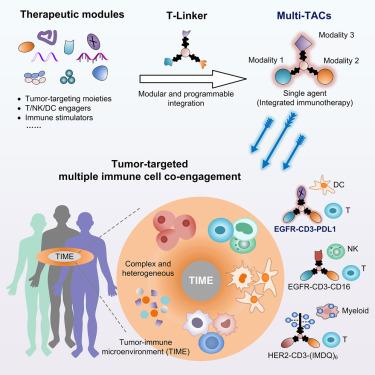
Multimodal targeting chimeras enable integrated immunotherapy leveraging tumor-immune microenvironment
Although immunotherapy has revolutionized cancer treatment, its efficacy is affected by multiple factors, particularly those derived from the complexity and heterogeneity of the tumor-immune microenvironment (TIME). Strategies that simultaneously and synergistically engage multiple immune cells in TIME remain highly desirable but challenging. Herein, we report a multimodal and programmable platform that enables the integration of multiple therapeutic modules into single agents for tumor-targeted co-engagement of multiple immune cells within TIME. We developed the triple orthogonal linker (T-Linker) technology to integrate various therapeutic small molecules and biomolecules as multimodal targeting chimeras (Multi-TACs). The EGFR-CD3-PDL1 Multi-TAC facilitated T-dendritic cell co-engagement to target solid tumors with excellent efficacy, as demonstrated in vitro, in several humanized mouse models and in patient-derived tumor models. Furthermore, Multi-TACs were constructed to coordinate T cells with other immune cell types. The highly modular and programmable feature of our Multi-TACs may find broad applications in immunotherapy and beyond.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


