发现吡咯并嘧啶酮衍生物作为治疗癌症的强效 PKMYT1 抑制剂
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
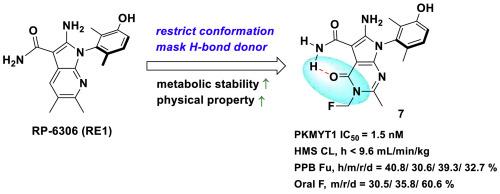
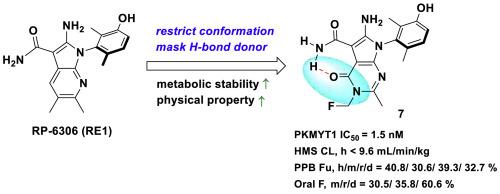
蛋白激酶 PKMYT1 负责抑制 CDK1 磷酸化,因此在调节 G2/M 细胞周期检查点方面发挥着核心作用。由于许多癌症都存在细胞周期检查点信号传导失调的问题,PKMYT1抑制剂正成为晚期肿瘤的一个诱人靶点。然而,PKMYT1 抑制剂在平衡生物有效性、靶向特异性、良好的稳定性和其他类药物特性方面遇到了困难。在此,我们报告了吡咯并嘧啶酮衍生物的设计和开发情况,这些衍生物旨在同时限制分子构象和保护代谢位点,以优化稳定性。化合物 7 在体外表现出很强的 PKMYT1 特异性抑制作用、CDK1 磷酸化随之降低和抗肿瘤功效,同时还具有更高的代谢稳定性、良好的药代动力学和生物利用度特性以及强大的体内抗肿瘤功效。我们的研究结果表明,化合物 7 是一种很有前景的 PKMYT1 抑制剂,可用于治疗存在细胞周期缺陷的晚期癌症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery of pyrrolopyrimidinone derivatives as potent PKMYT1 inhibitors for the treatment of cancer
The protein kinase PKMYT1 is responsible for inhibitory CDK1 phosphorylation, thus playing a central role in regulating the G2/M cell cycle checkpoint. As many cancers have dysfunctional cell cycle checkpoint signaling, PKMYT1 inhibition is emerging as an attractive target in advanced tumors. PKMYT1 inhibitors, however, have encountered difficulties in balancing biological efficacy, on-target specificity, and favorable stability and other drug-like properties. Herein, we report the design and development of pyrrolopyrimidinone derivatives intended to simultaneously restrict molecular conformation and shield a metabolic site in order to optimize stability. Compound 7 demonstrated strong PKMYT1-specific inhibition, a subsequent decrease in CDK1 phosphorylation, and antitumor efficacy in vitro, as well as enhanced metabolic stability, favorable pharmacokinetic and bioavailability properties, and potent antitumor in vivo efficacy. Our findings indicate that compound 7 is a promising PKMYT1 inhibitor for the treatment of advanced cancers with cell cycle defects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


