发现通过激活 PPARγ 治疗黑色素瘤的强效 HDAC 抑制剂氯喹衍生物
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
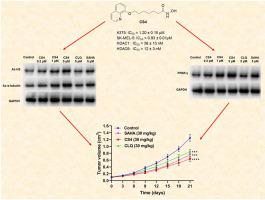
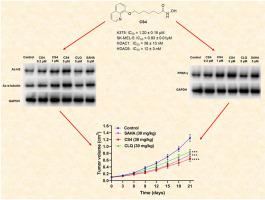
组蛋白去乙酰化酶(HDAC)抑制剂与过氧化物酶体增殖激活受体γ(PPARγ)激动剂联合治疗显示出显著的抗癌疗效。基于这些结果,研究人员制备了一系列氯喹衍生物,作为治疗黑色素瘤的强效 HDAC 抑制剂。在这些化合物中,CS4 对 HDAC1(IC50 = 38 nM)和 HDAC6(IC50 = 12 nM)有很好的抑制作用,对 A375 和 SK-MEL-5 黑色素瘤细胞有很好的抗增殖作用(IC50 值分别为 1.20 和 0.93 μM)。机理研究表明,CS4通过促进α-微管蛋白和组蛋白3(H3)乙酰化来抑制SK-MEL-5细胞的生长。在代谢水平上,BG11 能激活 PPARγ 并阻断 SK-MEL-5 细胞的糖酵解,从而介导 CS4 的部分抗黑色素瘤作用。此外,CS4 还能诱导细胞周期停滞在 G2 阶段,抑制迁移并促进 SK-MEL-5 细胞凋亡。更重要的是,与 SAHA 相比,化合物 CS4 在体内具有显著的抗癌效果,而且毒性可忽略不计。因此,CS4 是一种有效的 HDAC 抑制剂,可作为抗黑色素瘤的候选药物进行开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery of cloxiquine derivatives as potent HDAC inhibitors for the treatment of melanoma via activating PPARγ
The combined treatment with histone deacetylase (HDAC) inhibitors with peroxisome proliferator-activated receptor γ (PPARγ) agonists has displayed significant anticancer efficacy. Based on these results, a series of cloxiquine derivatives were prepared as potent HDAC inhibitors for the treatment of melanoma. Among these compounds, CS4 exhibited excellent inhibitory effects on HDAC1 (IC50 = 38 nM) and HDAC6 (IC50 = 12 nM), and had good antiproliferative effects against A375 and SK-MEL-5 melanoma cells (IC50 values, 1.20 and 0.93 μM, respectively). Mechanism research indicated that CS4 inhibited SK-MEL-5 cell growth by promoting α-tubulin and histone 3 (H3) acetylation. At the metabolic level, treatment with BG11 activated PPARγ and blocked glycolysis in SK-MEL-5 cells, which mediated partial antimelanoma effects of CS4. In addition, CS4 also induced cell cycle arrest at G2, suppressed migration and facilitated apoptosis of SK-MEL-5 cells. More importantly, compound CS4 demonstrated significant in vivo anticancer effect compared with SAHA, and exhibited neglectable toxicity. Consequently, CS4 is the potent HDAC inhibitor, which may be developed as the candidate antimelanoma drug.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


