泛基因组分析揭示了金针菇驯化过程中的基因组变异,重点是菌盖颜色和菌柄长度的遗传特征
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
引言食用菌(包括丝状蘑菇)的驯化为人工选择驱动的基因变化提供了宝贵的见解。本研究旨在调查丝状蘑菇的种群结构、遗传多样性以及与驯化相关的基因组变化。通过比较 199 株野生菌株和栽培菌株的基因组序列,我们旨在阐明驯化对丝状蘑菇的影响。方法我们对 199 株菌株进行了全新基因组组装和基于基因的泛基因组分析,其中包括野生菌株和栽培菌株。我们还利用存在-不存在变异(PAV)和SNP数据并结合RNA测序进行了全基因组关联研究(GWAS),以确定与驯化性状(如蘑菇伞颜色和茎秆长度)相关的基因。通过遗传转化实验确认了基因的功能。结果我们的分析将菌株分为四个不同的群体,它们与不同强度的人工选择相关。与野生种群相比,三个栽培种群的基因组规模较小,基因数量较少,遗传变异较低,基因表达多样性降低,杂合度较低。分析表明,在驯化过程中,与β-内酰胺类抗生素分解代谢过程相关的基因和特定的MAPK通路基因丢失,使驯化品系更易感染疾病。本研究发现了栽培和野生丝状藻种群之间的显著基因组变异,包括驯化过程中病原体抗性基因的丢失。我们还发现了与菌盖颜色和菌柄长度相关的关键基因,首次证明了 PAV 变异在蘑菇驯化过程中的重要作用。这些见解为未来的蘑菇育种和进化研究奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
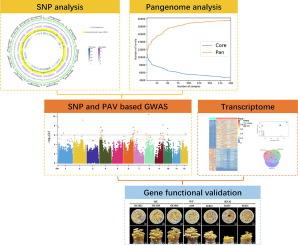
Pan-genome analysis reveals genomic variations during enoki mushroom domestication, with emphasis on genetic signatures of cap color and stipe length
Introduction
The domestication of edible mushrooms, including Flammulina filiformis, offers valuable insights into the genetic changes driven by artificial selection. Understanding these changes is crucial for uncovering the mechanisms behind genome evolution in domesticated mushrooms.Objectives
This study aims to investigate the population structure, genetic diversity, and domestication-related genomic changes in F. filiformis. By comparing the genome sequences of 199 wild and cultivated strains, we aim to elucidate the impact of domestication on F. filiformis.Methods
We performed de novo genome assembly and gene-based pan-genome analysis on the 199 strains, which included both wild and cultivated strains. We also conducted genome-wide association studies (GWAS) using presence-absence variation (PAV) and SNP data, combined with RNA sequencing, to identify genes associated with domestication traits, such as cap color and stalk length. Gene functional confirmation was achieved through genetic transformation experiments.Results
Our analysis grouped the strains into four distinct populations, which correlated with varying intensities of artificial selection. The three cultivated populations exhibited smaller genome sizes, fewer genes, lower genetic variation, reduced gene expression diversity, and lower heterozygosity compared to the wild population. The analysis revealed the loss of genes related to the beta-lactam antibiotic catabolic process and specific MAPK pathway genes during domestication, rendering domesticated strains more susceptible to diseases. Four genes closely associated with cap color and stipe length were identified, but genetic transformation experiments confirmed the functional relevance of only two (FfB and FfD) identified through PAV-based GWAS.Conclusion
This study uncovered significant genomic variations between cultivated and wild Flammulina filiformis populations, including the loss of pathogen resistance genes during domestication. We also identified key genes linked to cap color and stipe length, demonstrating for the first time the important role of PAV variation in mushroom domestication. These insights provide a foundation for future mushroom breeding and evolutionary research.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


