通过外螺旋霍兹平面吸附调节实现与石墨阳极兼容的低浓度纯碳酸丙烯酯溶剂电解质
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
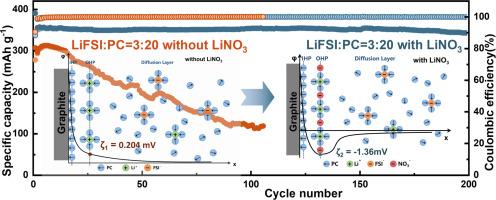
碳酸丙烯酯(PC)的低熔点和高极性使其成为锂离子电池(LIB)电解质的理想溶剂,但与石墨负极的不相容性阻碍了其应用。本研究开发了一种新方法,从新的角度解决了这一问题。在含有正常浓度(1.38 mol L-1)锂盐的纯 PC 电解液中引入饱和 LiNO3(0.14 mol L-1),可与石墨负极完美兼容。使用这种纯 PC 电解液的锂/石墨电池显示出 350.0 mAh g-1 的可逆容量和 96.3% 的 200 周期容量保持率。进一步的机理研究和理论计算表明,NO3- 阴离子吸附在石墨阳极界面电双层的外赫尔姆霍兹平面上。这改变了电双层中 Li+ 的溶解结构,促进了 Li+ 的解溶解过程,因此与石墨阳极具有很高的兼容性。本策略最突出的优点是,由于 NO3- 阴离子吸附在外赫尔姆霍兹平面上,因此只需引入极少量的 Li 盐,就能提高与石墨阳极的相容性,而不会对电解质的体相性质产生较大的变化。这项研究从外赫尔姆霍兹面的角度提供了对相容性的新认识,可能为优化锂离子电池电解质提供新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Outer Helmholtz plane adsorption regulation to achieve low-concentration of pure propylene carbonate solvent electrolytes compatible with graphite anode
The low melting point and high polarity of propylene carbonate (PC) make it an ideal solvent for electrolytes of lithium-ion batteries (LIBs), but the incompatibility with graphite anode hinders the application. In the present study, a new method is developed to solve this problem from a new viewpoint. Introduction of saturated LiNO3 (0.14 mol L‒1) into pure PC electrolyte containing lithium salt with normal concentration (1.38 mol L‒1) leads to a perfect compatibility with graphite anode. Li/Graphite cell using this pure PC electrolyte shows a reversible capacity of 350.0 mAh g‒1 and a 200-cycle capacity retention of 96.3 %. Further mechanism study and theoretical computation indicate that the NO3‒ anions are adsorbed to outer Helmholtz plane of the electric double layer at the graphite anode interface. This changes the Li+ solvation structure in the electric double layer, facilitating the Li+ desolvation process hence resulting in high compatibility with the graphite anode. The most prominent advantage of the present strategy is that, because of the adsorption of NO3‒ anions to the outer Helmholtz plane, the compatibility with graphite anode could be improved by introduction of tiny amount of Li salt without large change of the bulk phase properties of the electrolyte. This work provides a new understanding of the compatibility from the viewpoint of outer Helmholtz plane, which might deliver new insights for the optimization of electrolytes for LIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


