Rh(III)-Catalyzed Alkene Anti Nucleoamidation to Access Diverse Heterocycles(Rh(III)催化烯反核氨基化以获得多种杂环
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
烯烃双官能化方法是一种极具吸引力的方法,可利用现成的起始材料快速构建复杂的分子。一种常见的方法是过渡金属催化的核金属化,它将 C-C 双键转化为 C-Nu 键和 C-M 键。通常情况下,这种方法会使用一个系链亲核体来形成与制药相关的杂环核心。生成的烷基金属可通过多种方式进一步官能化。虽然存在酰胺化 C-M 键的方法,但直接安装有价值的酰胺却很少见。此外,核金属化方法通常仅限于使用单一类型的亲核体。在此,我们公开了一种 Rh(III) 催化的烯烃核酰胺化通用方法,可形成多种杂环核心。本文章由计算机程序翻译,如有差异,请以英文原文为准。
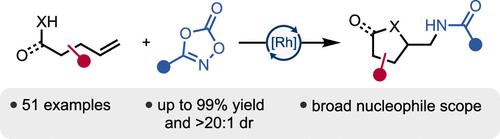
Rh(III)-Catalyzed Alkene Anti Nucleoamidation to Access Diverse Heterocycles
Alkene difunctionalization methods are an attractive way to rapidly build molecular complexity by using readily available starting materials. One common approach is transition metal-catalyzed nucleometalation, which converts the C–C double bond to a C–Nu bond and a C–M bond. Commonly, a tethered nucleophile is used to form pharmaceutically relevant heterocyclic cores in this manner. The resulting alkylmetal species can then be further functionalized in a number of ways. While methods exist to aminate the C–M bond, direct installation of valuable amides is rare. Additionally, nucleometalation methods are often limited to the use of a single type of nucleophile. Herein, we disclose a general method for the Rh(III)-catalyzed nucleoamidation of alkenes, forming a variety of heterocyclic cores.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


