TRPM2介导的前馈环通过钙-cGAS-STING-NF-κB通路促进骨关节炎中软骨细胞的损伤
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
引言骨关节炎(OA)是导致老年人残疾的一个重要因素。然而,目前的治疗方案十分有限。方法收集骨关节炎(OA)患者和OA小鼠的软骨样本,检测TRPM2的表达水平。为了研究调节 TRPM2 对小鼠内侧半月板失稳(DMM)诱导的膝关节 OA 的影响,我们利用 TRPM2 基因敲除小鼠和腺病毒介导的 TRPM2 过表达。 此外,我们还进行了 siRNA 介导的 TRPM2 基因敲除或质粒介导的 TRPM2 过表达,以探讨 TRPM2 在 IL-1β 诱导的软骨细胞中的作用。通过信号通路抑制剂筛选了IL-1β对TRPM2表达的调控机制,并利用数据库预测了TRPM2的转录因子和结合位点。通过芯片-PCR和ChIP-qPCR验证了RELA(NF-κB-p65)与Trpm2启动子的结合。结果 在 OA 患者和 OA 小鼠的软骨中观察到 TRPM2 的表达增加。此外,缺乏 TRPM2 的小鼠对 DMM 诱导的 OA 进展具有保护作用。与此相反,TRPM2的过表达会导致DMM诱导的OA恶化,并促进软骨细胞OA样表型的形成。IL-1β以NF-κB-p65依赖的方式上调TRPM2。随后,TRPM2-Ca2+-mtDNA-cGAS-STING-NF-κB轴在OA进展中的作用得到了验证。此外,用 BAPTA-AM 抑制 TRPM2-Ca2+ 轴可有效减轻已形成的 OA。结论我们的数据共同揭示了 OA 软骨细胞中涉及 TRPM2、Ca2+、mtDNA、cGAS、STING 和 NF-κB 的病理反馈回路。这表明,破坏这一环路可能是治疗 OA 的一种可行方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
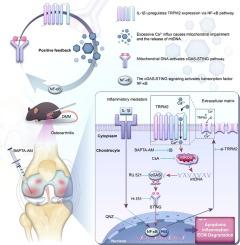
TRPM2-mediated feed-forward loop promotes chondrocyte damage in osteoarthritis via calcium-cGAS-STING-NF-κB pathway
Introductions
Osteoarthritis (OA) is a significant contributor to disability in the elderly population. However, current therapeutic options are limited. The transient receptor potential melastatin 2 (TRPM2) is involved in a range of disease processes, yet its role in OA remains unclear.Objectives
To investigate the role of TRPM2 in OA.Methods
Cartilage samples were collected from patients with osteoarthritis (OA) and mice with OA to examine TRPM2 expression levels. To investigate the effects of TRPM2 modulation on the destabilization of the medial meniscus (DMM) induced knee OA in mice, we utilized TRPM2 knockout mice and employed adenovirus-mediated overexpression of TRPM2. Furthermore, siRNA-mediated TRPM2 knockdown or plasmid-mediated TRPM2 overexpression was conducted to explore the role of TRPM2 in IL-1β-induced chondrocytes. The regulatory mechanism of IL-1β on TRPM2 expression was screened by signaling pathway inhibitors, and the transcription factors and binding sites of TRPM2 were predicted using the database. The binding of RELA (NF-κB-p65) to the Trpm2 promoter was verified by chip-PCR and ChIP-qPCR. The therapeutic potential of Ca2+ chelation with BAPTA-AM for the treatment of osteoarthritis (OA) was investigated.Results
An increased expression of TRPM2 was observed in the cartilage of OA patients and OA mice. Furthermore, mice deficient in Trpm2 exhibited a protective effect against DMM-induced OA progression. In contrast, TRPM2 overexpression resulted in exacerbation of DMM-induced OA and the promotion of an OA-like phenotype of chondrocytes. TRPM2 was upregulated by IL-1β in an NF-κB-p65-dependent manner. Subsequently, the TRPM2-Ca2+-mtDNA-cGAS-STING-NF-κB axis in the progression of OA was validated. Furthermore, inhibition of the TRPM2-Ca2+ axis with BAPTA-AM effectively attenuated established OA.Conclusions
Our data collectively revealed a pathological feedback loop involving TRPM2, Ca2+, mtDNA, cGAS, STING, and NF-κB in OA chondrocytes. This suggests that disrupting this loop could be a viable therapeutic approach for OA.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


