框架外氢化镓物种被困在 MFI 沸石中的铝对上进行丙烷脱氢的理论研究
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
Ga/ZSM-5 广泛用于催化丙烷脱氢(PDH)。在苛刻的操作条件下,Ga/ZSM-5 的详细活性物种以及导致观察到的卓越 PDH 性能的合理反应途径仍未得到解决。滞留在沸石框架 Al 对上的还原框架外 Ga 氢化物物种,包括 b[GaH]2+、[Ga]+-BAS、[GaH2]+-BAS 等,是一种重要的 Ga 物种,被称为 PDH 的活性物种。在这项工作中,我们在与实验相关的温度和压力下对这些镓物种的 PDH 途径进行了理论研究。我们发现,与其他氢化镓物种相比,动态生成的欠配位 b[GaH]2+ 在 PDH 条件下会表现出更高的催化活性。b[GaH]2+ 最合理的 PDH 途径是硒途径。Ga 物种和 BAS 在促进 H2 消解和 β-H 转移以及提高 PDH 效率方面发挥着协同作用。镓氢化物物种也可能作为中间体沿着 PDH 途径形成,作为随后活化和转化丙烷的活性位点,这表明在 Ga/ZSM-5 的操作条件下,镓氢化物物种被 Al 对所捕获。这些发现有望为更好地理解实验观察到的 Ga/ZSM-5 与操作条件有关的 PDH 性能铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
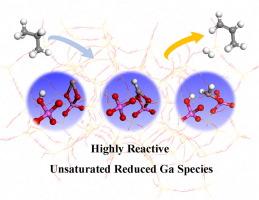
Theoretical investigation on propane dehydrogenation over extraframework Ga hydride species trapped at Al-pairs in MFI zeolite
Ga/ZSM-5 is widely used to catalyze propane dehydrogenation (PDH). For the harsh operation conditions, the detailed active species, and the plausible reaction pathways that are responsible for the observed superior PDH performance remain unresolved for Ga/ZSM-5. Reduced extraframework Ga hydride species trapped at zeolite framework Al-pairs, including b[GaH]2+, [Ga]+-BAS, [GaH2]+-BAS, etc. are an important kind of Ga species known as active species for PDH. In this work, the PDH pathways these over these Ga species were investigated theoretically at temperatures and pressures relevant to experiments. We showed that dynamically generated undercoordinated b[GaH]2+ would exhibit significantly higher catalytic activity at PDH conditions as compared with other Ga hydride species. The most plausible PDH pathway over b[GaH]2+ is the carbenium pathway. The Ga species and BAS act in concert in promoting the H2 elimination and β-H transfer, and in turning PDH efficient. Ga hydride species may also form as intermediates along the PDH pathways as active sites for subsequent activation and conversion of propane, suggesting the evolution of Ga hydride species trapped by Al-pairs in Ga/ZSM-5 at operation conditions. The findings are expected to pave the way for better understanding of the experimentally observed operation condition dependent PDH performance of Ga/ZSM-5.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


