铜催化有氧重排直接合成 N-融合吲哚
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
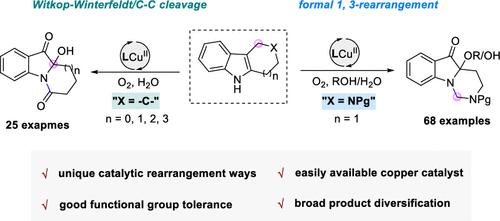
N-融合吲哚是典型的 N-杂环,广泛存在于天然产物和生物活性分子中。尽管它们非常重要,但 N-融合吲哚的合成尚未得到充分发展。在此,我们报告了一种直接的、通用的统一铜催化有氧骨架重排策略,该策略使用现成的环状吲哚底物,为快速构建各种 N-融合吲哚支架提供了一个实用的合成平台。这种开瓶方法反应条件温和,化学选择性高,底物范围广(超过 90 个实例)。使用不同的亲核剂对产物进行放大合成和多功能转化,证明了该方法的可扩展性和实用性。机理研究表明,通过独特的单电子转移(SET)诱导的有氧加氧机理,四氢-γ-咔啉的反应是通过正式的 1,3 迁移重排进行的,而四氢咔唑的反应则是通过 Witkop-Winterfeldt/C-C 裂解级联进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Direct Synthesis of N-Fused Indoles Enabled by Copper-Catalyzed Aerobic Oxygenative Rearrangement
N-Fused indoles are typical N-heterocycles, which are extensively found in natural products and bioactive molecules. Despite their importance, the synthesis of N-fused indoles has not yet been fully developed. Herein, we report a direct, general unified copper-catalyzed aerobic oxygenative skeletal rearrangement strategy using readily available cyclic indole substrates, which provides a practical synthetic platform for the rapid construction of a wide array of N-fused indole scaffolds. This open-flask method features mild reaction conditions, high chemoselectivity, and a broad substrate scope (over 90 examples). The scaled-up synthesis and versatile transformations of the products using various nucleophiles demonstrated the scalability and utility of this protocol. Mechanistic studies revealed that, by involving a unique single-electron transfer (SET)-induced aerobic oxygenation mechanism, the reaction of tetrahydro-γ-carbolines proceeded via a formal 1,3-migration rearrangement, while that of tetrahydrocarbazoles proceeded by a Witkop–Winterfeldt/C–C cleavage cascade.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


