视黄醇结合蛋白 4 通过选择性自噬降解病毒 ORF1 蛋白限制 PCV2 复制
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
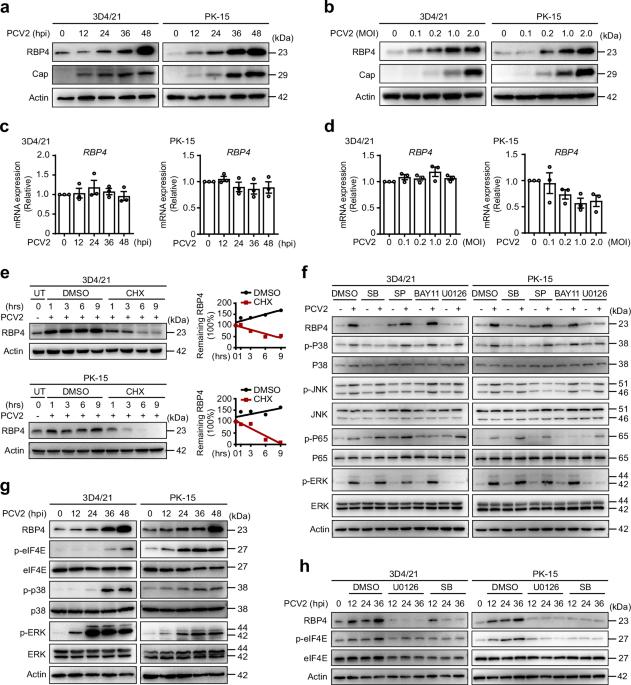
自噬是一种高度保守的降解过程,与多种功能有关,包括保护宿主细胞免受病原体感染。虽然自噬在猪圆环病毒 2(PCV2)感染中的参与已变得很明显,但选择性自噬是否在 PCV2 限制中发挥关键作用仍不清楚。在这里,我们发现视黄醇结合蛋白 4(RBP4)是一种视黄醇载体脂肪因子,它能启动 PCV2 ORF1 蛋白的自噬降解。在活细胞中,PCV2 感染会通过 MAPK-eIF4E 轴增加 RBP4 蛋白水平。异位表达 RBP4 或重组 RBP4 可促进 ORF1 蛋白的降解。从机制上讲,RBP4激活TRAF6诱导ORF1发生K63连接的泛素化,从而导致SQSTM1/p62介导的选择性自噬降解。因此,RBP4 缺乏会增加病毒载量并加剧 PCV2 在体内的致病性。总之,这些结果确定了 RBP4 是 PCV2 的一个关键宿主限制因子,并揭示了一种以前未曾描述过的在受感染细胞中对抗 PCV2 的抗病毒机制。视黄醇结合蛋白4激活TRAF6,通过SQSTM1/p62介导的选择性自噬诱导K63连接的泛素化和降解PCV2 ORF1蛋白,从而限制PCV2的复制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Retinol binding protein 4 restricts PCV2 replication via selective autophagy degradation of viral ORF1 protein
Autophagy is a highly conserved degradative process that has been linked to various functions, including defending host cells against pathogens. Although the involvement of autophagy in porcine circovirus 2 (PCV2) infection has become apparent, it remains unclear whether selective autophagy plays a critical role in PCV2 restriction. Here we show that retinol-binding protein 4 (RBP4), an adipokine for retinol carrier, initiates the autophagic degradation of PCV2 ORF1 protein. PCV2 infection increases RBP4 protein levels through MAPK-eIF4E axis in living cells. Ectopic expression of RBP4 or recombinant RBP4 treatment promotes the degradation of ORF1 protein. Mechanistically, RBP4 activates TRAF6 to induce K63-linked ubiquitination of ORF1, leading to SQSTM1/p62-mediated selective autophagy for degradation. Consequently, RBP4 deficiency increases viral loads and exacerbates the pathogenicity of PCV2 in vivo. Collectively, these results identify RBP4 as a key host restriction factor of PCV2 and reveal a previously undescribed antiviral mechanism against PCV2 in infected cells. Retinol binding protein 4 activates TRAF6 to induce K63-linked ubiquitination and degradation of PCV2 ORF1 protein through SQSTM1/p62-mediated selective autophagy to restrict PCV2 replication.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


