使用轮胎炭催化剂对废轮胎热解挥发物进行催化蒸汽转化,以获得高产率的富氢合成气
IF 7.2
2区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
研究人员在两级固定床反应器中研究了利用热解催化蒸汽转化法从废轮胎中生产氢气和合成气(H2/CO)的问题。在这项研究中,轮胎炭作为牺牲催化剂,促进了催化蒸汽转化和炭蒸汽气化反应的结合。轮胎炭既是催化剂又是气化反应物,从而提高了气体产品的产量。研究的工艺参数包括:重整温度范围为 700-1000 °C,蒸汽空间速度为 2-12 g h-1 g-1char,反应时间为 0.5-2 h。结果表明,在使用轮胎炭催化剂的情况下,较高的温度和蒸汽空间速度会提高 H2 和 CO 的产率。对使用过的轮胎炭的元素分析、表面形态和孔隙结构有助于了解轮胎炭在反应中的消耗程度。延长反应时间可使热解挥发物与轮胎炭之间的反应更加彻底,从而促进 H2 的产生。在反应时间为 2 小时时,H2 产量达到 223 mmol g-1,占最大氢产量的 74 wt%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
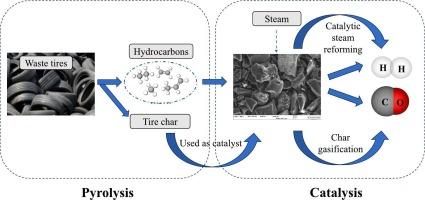
Catalytic steam reforming of waste tire pyrolysis volatiles using a tire char catalyst for high yield hydrogen-rich syngas
The production of hydrogen and syngas (H2/CO) from waste tires by pyrolysis catalytic steam reforming was investigated in a two-stage fixed bed reactor. In this study, tire char served as a sacrificial catalyst, facilitating the combination of catalytic steam reforming and char steam gasification reactions. The tire char acted as both a catalyst and a gasification reactant, enhancing the gas product yield. The process parameters investigated were, a reforming temperature range of 700–1000 °C, steam space velocity between 2 and 12 g h−1 g−1char and reaction times of 0.5–2 h. The influence of the parameters on the yield and composition of the product gases and the characteristics of the used catalyst were analyzed in detail. The results indicated that higher temperature and steam space velocity increased H2 and CO yields in the presence of a tire char catalyst. Elemental analysis of the used tire char, surface morphology and pore structure provided insights into the extent of tire char consumption in the reaction. Prolonged reaction time allowed for more thorough reactions between the pyrolysis volatiles and tire char, promoting the production of H2. At a reaction time of 2 h, the H2 yield reached 223 mmol g−1, representing 74 wt% of the maximum hydrogen yield.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel Processing Technology
工程技术-工程:化工
CiteScore
13.20
自引率
9.30%
发文量
398
审稿时长
26 days
期刊介绍:
Fuel Processing Technology (FPT) deals with the scientific and technological aspects of converting fossil and renewable resources to clean fuels, value-added chemicals, fuel-related advanced carbon materials and by-products. In addition to the traditional non-nuclear fossil fuels, biomass and wastes, papers on the integration of renewables such as solar and wind energy and energy storage into the fuel processing processes, as well as papers on the production and conversion of non-carbon-containing fuels such as hydrogen and ammonia, are also welcome. While chemical conversion is emphasized, papers on advanced physical conversion processes are also considered for publication in FPT. Papers on the fundamental aspects of fuel structure and properties will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


