光学纯反式-2-(3,5-二甲基苯氧基)环己-1-醇作为制备α-取代羧酸衍生物的手性助剂的合成与应用
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究合成了反式-2-(3,5-二甲基苯氧基)环己-1-醇,并通过酶促动力学解析(EKR)分离了其对映体。通过使用已知手性描述的酯转化成非对映异构体,确定了绝对构型。然后用手性醇作为辅助剂,在 DCC 和适当的碱存在下,通过与外消旋α-卤酸偶联制备酯。我们观察到一种高效的动态动力学解析,因为产物酯是以非对映形式富集得到的。我们研究了碱、温度和反应时间等参数的影响。通过计算分析研究了非对映异构体的相对能量曲线,非对映产物的能量差与观察到的非对映选择性相符。手性纯的α-卤酸可以从助剂中分离出来,而不会损失两种成分的光学纯度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
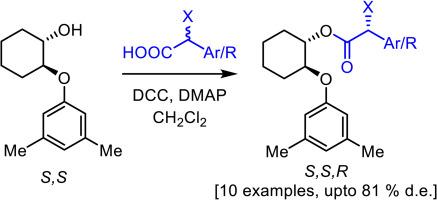
Synthesis and applications of optically pure trans-2-(3,5-dimethylphenoxy)cyclohexan-1-ol as chiral auxiliary for preparation of α-substituted carboxylic acid derivatives
In the present work, trans-2-(3,5-dimethylphenoxy)cyclohexan-1-ol was synthesized and its enantiomers were separated by Enzymatic Kinetic Resolution (EKR). The absolute configuration was established by converting to diastereomer with esters of known chiral description. Chiral alcohol was then used as an auxiliary for the preparation of esters by coupling with racemic α-halo acids in presence of DCC and a suitable base. We observed an efficient Dynamic Kinetic Resolution as the product esters were obtained in enriched diastereomeric forms. Effect of several parameters like base, temperature and reaction time was studied. The relative energy profile of the diastereomers was studied by computational analysis and the energy difference of diastereomeric products were in accordance with the observed diastereoselectivity. The chirally pure α-halo acid could be separated from the auxiliary, without any loss of optical purity of both components.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


