核苷酸配位聚合物是一种基于 ROS 的免疫调节抗菌剂,可加倍杀死植入感染的铜绿假单胞菌生物膜
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
铜绿假单胞菌是导致高发病率和高死亡率的病原菌,几十年来新批准的抗生素一直在减少。我们采用了一种绿色、通用的去质子化驱动策略来筛选鸟苷酸-金属离子配位聚合物纳米粒子(GMC),而不是在特定金属离子参与时发生结合失败。我们发现,GMC-2 精确的 pH 依赖性氧化酶样活性为铜绿假单胞菌生物膜感染的免疫调节谱写了一曲双交响乐。具体来说,GMC-2 介导的活性氧(ROS)调节会引发线粒体功能障碍,并释放与损伤相关的分子模式,与模式识别受体结合,导致内源性先天性免疫激活。同时,在微酸性生物膜环境中,GMC-2 触发的 ROS 生成会破坏生物膜,暴露出外源性病原体相关分子模式。与传统抗生素相比,GMC-2 不会导致铜绿假单胞菌产生抗药性。在受感染的植入小鼠模型中,GMC-2 介导的先天性免疫和适应性免疫可有效消除铜绿假单胞菌生物膜。这些发现提供了一种促进生物分子与金属离子结合的通用方法,并强调了精确的 ROS 调节平台在启动针对细菌生物膜感染的内源性和外源性免疫激活中的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
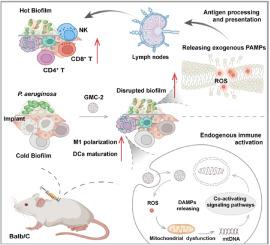
Nucleotide coordinated polymers, a ROS-based immunomodulatory antimicrobial, doubly kill Pseudomonas aeruginosa biofilms of implant infections
Pseudomonas aeruginosa causes high morbidity and mortality in nosocomial infections, and newly approved antibiotics have been declining for decades. A green and universal deprotonation-driven strategy is used to screen the guanylic acid-metal ion coordination polymer nanoparticles (GMC), instead of the failure of binding occurs when specific metal ion participation. We find that the precise pH-dependent oxidase-like activity of GMC-2 orchestrates a duple symphony of immune modulation for Pseudomonas aeruginosa biofilm infections. Specifically, GMC-2-mediated reactive oxygen species (ROS) regulation triggers mitochondrial dysfunction and releases damage-associated molecular patterns, engaging pattern recognition receptors and resulting in endogenous innate immune activation. Meanwhile, GMC-2-triggered ROS generation in a mildly acidic biofilm environment destroys the biofilm, exposing exogenous pathogen-associated molecular patterns. GMC-2 cannot cause resistance for Pseudomonas aeruginosa compared with conventional antibiotics. In an infected implant mouse model, Pseudomonas aeruginosa biofilms were effectively eliminated by GMC-2-mediated triggering of innate and adaptive immunity. These findings provide a universal approach for facilitating the binding of biomolecules with metal ions and highlight the precise ROS-regulating platform plays a critical role in initiating endogenous and exogenous immune activation targeted for bacterial biofilm infection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


