使用脒肟功能化碳毡一步式电化学还原和沉淀去除酸性废水中的六价铬
IF 8.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
传统的电化学方法处理酸性含铬废水往往面临着效率低、电极腐蚀和产生污泥等各种挑战。本研究提出了一种电化学还原结合原位沉淀的新方法来克服这些弊端,并利用脒肟功能化电极来提高六价铬的去除效率。研究还考察了 pH 值和电流密度的影响以及电极的可重复使用性。结果表明,由于脒肟基团的亲水性和正电效应,碳毡阴极上的 Cr(VI) 电化学还原率从 39.5% 提高到 99.2%。同时,即使在酸性溶液中,生成的 Cr(III) 也会在阴极上水解沉淀,从而使 Cr(T) 去除率达到 87.4%。电化学 H2O 分裂和 O2 还原创造了碱性溶液的局部环境,Cr(OH)3 在其中变得过饱和。溶液酸度(pH 值为 1.75-3.00)和外加电流密度(0-12.5 A m-2)的增加加快了 Cr(VI) 的还原速度,但先增加后降低了 Cr(III) 的阴极析出率。通过使用稀盐酸溶解绝缘沉淀物,电极可以重复使用和再生。这项研究为从酸性废水中去除六价铬提供了一种前景广阔的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
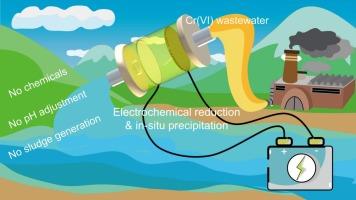
One-step electrochemical reduction and precipitation removal of Cr(VI) in acid wastewaters using amidoxime-functionalized carbon felt
Traditional electrochemical methods to treat acidic chromium-containing wastewater are often faced with various challenges such as low efficiency, electrode corrosion, and sludge generation. This study proposed a novel method of electrochemical reduction coupled with in-situ precipitation to overcome these drawbacks, and utilized amidoxime-functionalized electrodes to enhance Cr(VI) removal efficiency. The influence of pH and current density and the electrode reusability were also investigated. The results showed that the electrochemical reduction ratio of Cr(VI) on carbon felt cathode increased from 39.5 % to 99.2 % after functionalization with amidoxime groups due to their hydrophilicity and positive electric effect. Concurrently, the generated Cr(III) was hydrolytically precipitated on the cathode even in an acidic bulk solution, resulting in a Cr(T) removal ratio of 87.4 %. Electrochemical H2O-splitting and O2 reduction created a local environment of alkaline solution, where Cr(OH)3 became supersaturated. Increases in both the solution acidity (pH 1.75–3.00) and applied current density (0–12.5 A m−2) accelerated the reduction rate of Cr(VI), but first increased and then decreased the cathodic precipitation ratio of Cr(III). The electrodes could be repeatedly reused and regenerated through dissolving the insulated precipitates using diluted hydrochloric acid. This study provides a promising strategy to remove Cr(VI) from acidic wastewaters.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


