评估麦角硫因与人血清白蛋白的结合机制:多光谱分析、分子对接和分子动力学模拟
IF 4.3
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-11-03
DOI:10.1016/j.saa.2024.125368
引用次数: 0
摘要
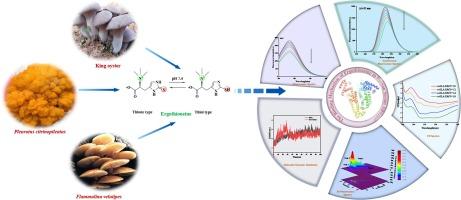
麦角硫因(EGT)具有极强的抗氧化性,被公认为 "长寿维生素",因而备受关注。为了全面了解麦角硫因的药效学和药代动力学,我们通过先进的多光谱分析方法和结合分子动力学模拟的硅学分子对接,阐明了麦角硫因与人血清白蛋白(HSA)的结合机制。荧光淬灭结果表明,EGT 与 HSA 的结合是一种静态淬灭模式,其降序 Stern-Volmer 常数(Ksv)值(2.82、2.36、1.48 × 104 L mol-1)和生物分子淬灭速率常数(Kq)值(2.van't Hoff 标准显示 EGT 与 HSA 的结合是一个自发的放热过程(ΔG = -24.紫外可见吸收光谱、同步荧光光谱、三维荧光光谱和圆二色性分析表明,EGT 的加入影响了 Trp214 的微环境,并重新排列了 HSA 的结构。结合置换分析表明,它们的结合位点靠近 HSA 的亚域 IIA(Sudlow 位点 I),这在分子对接中得到了直观的显示。除了明显的范德华力、吸引电荷和 Pi-alkyl 相互作用外,EGT 侧链中的手性甜菜碱基团(N+(CH3+)3)倾向于与白蛋白疏水空腔中的 Lys199、Ser287 和 Arg257 形成氢键。此外,动态模拟还通过四项指标(RMSD、RMSF、Rg、SASA)证实了在100 ns内形成的对接复合物的平衡性和稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Assessment of the binding mechanism of ergothioneine to human serum albumin: Multi-spectroscopy, molecular docking and molecular dynamic simulation
Ergothioneine (EGT) has attracted great attention due to its extremely potent antioxidant properties, universally acknowledged as ‘longevity vitamin’. In order to comprehensive understanding of its pharmacodynamics and pharmacokinetics, the binding mechanism of EGT with human serum albumin (HSA) was clarified by cutting-edged multi-spectroscopic approaches and in silico molecular docking coupled with molecular dynamic simulation. Our fluorescence quenching results revealed that the binding of EGT to HSA was in a static quenching mode validated by the descending Stern–Volmer constant (Ksv) values (2.82, 2.36, 1.48 × 104 L mol−1) and biomolecular quenching rate constant (Kq) values (2.82, 2.36, 1.48 × 1012 L mol−1) at 298 K, 305 K, and 310 K, respectively. van’t Hoff criterion revealed the combination of EGT with HSA was a spontaneous exothermic process (ΔG = −24.16 kJ mol−1) via hydrogen bonding and van der Waals force interactions (ΔH = −60.25 kJ mol−1, ΔS = −129.44 J mol−1 K−1) at 310 K. The analysis of UV–vis absorption spectrum, synchronous fluorescence spectrum, three-dimensional fluorescence spectrum and circular dichroism indicated the addition of EGT affected the microenvironment of Trp214 and rearranged the structure of HSA. The binding replacement assay interpreted their binding site was near the subdomain IIA of HSA (Sudlow’s site I), which was intuitively exhibited by molecular docking. In addition of obvious van der Wall forces, attractive charge and Pi-alkyl interactions, the chiral betaine group (N+(CH3+)3) in the side chain of EGT was inclined to form hydrogen bonds with Lys199, Ser287 and Arg257 in the hydrophobic cavity of albumin. Moreover, the dynamic simulation reinforced the equilibrium and stability of formed docking complex by four indicators (RMSD, RMSF, Rg, SASA) within 100 ns.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


