化学驱动的淀粉样蛋白清除,用于阿尔茨海默病的治疗和诊断。
摘要
展望 一个世纪前,德国神经学家阿洛伊斯-阿尔茨海默(Alois Alzheimer)记录了第一例阿尔茨海默病(AD)病例,揭示了与大脑中存在异常蛋白质群(包括淀粉样蛋白斑块和tau缠结)有关的认知障碍。在典型的生理状态下,淀粉样蛋白-β(Aβ)的水平保持平衡,但衰老会因Aβ的过度生成、清除障碍和其他因素而破坏Aβ的平衡,最终导致Aβ的积累。尽管 Aβ 聚集与神经变性之间的联系使 Aβ 长期以来一直是治疗注意力缺失症的一个有希望的靶点,但数十年来针对 Aβ 的药物开发一直未获成功,因此人们对这一策略治疗注意力缺失症的疗效持怀疑态度。然而,美国食品及药物管理局最近批准的抗Aβ抗体药物,包括阿杜单抗(aducanumab,Aduhelm)、莱卡内单抗(lecanemab,Leqembi)和多那尼单抗(donanemab,Kisunla),促使人们重新评估这一观点。这些疗法已证明能有效降低脑 Aβ 水平,从而减缓疾病进展,并再次证实 Aβ 是一个关键靶点。尽管取得了进步,但免疫疗法也存在相当多的缺点,包括不良反应、高昂的成本和繁琐的管理。为了解决这些局限性,我们的研究重点是开发既能减轻抗体疗法的挑战,又能提供实用、方便选择的小分子药物。我们发现 4-(2-羟乙基)-1-哌嗪丙磺酸(EPPS)是一种很有前景的化合物,它能显著减少大脑中聚集的 Aβ,并增强 AD 啮齿动物模型的行为。给药后,EPPS 可穿透血脑屏障(BBB)并与有毒的 Aβ 聚集体结合,随后将其分解为无毒的单体。这将产生两个重要结果:减少大脑中的 Aβ 聚集体,随后增加血液中的 Aβ 单体。与聚集形式不同的 Aβ 单体现在可以穿过 BBB 进入血液。这种机制为注意力缺失症的治疗和诊断提供了一种创新方法。通过分离脑Aβ聚集体,EPPS促进了Aβ的清除,解决了AD的一个关键病理特征。同时,血液中 Aβ 水平的升高为监测治疗效果和疾病进展提供了潜在的生物标志物,从而彻底改变了注意力缺失症的治疗和诊断。研究候选药物的详细作用模式需要目标蛋白质的结构信息。遗憾的是,Aβ聚集体具有不稳定性和异质性,会形成较大的团块,这使得这些结构的鉴定变得复杂。因此,我们开发了筛选小分子的新工具,将单体 Aβ 及其片段固定在平板上。这使我们不仅能鉴定出以 Aβ 为靶点的新型化合物,还能阐明它们的作用机制,从而开发出以 Aβ 为靶点的 AD 治疗方法。我们相信,我们在通过小分子化学驱动淀粉样蛋白清除方面所做的工作代表了注意力缺失症研究的一个进步,它提供了化学多样性和直接、经济的开发过程。在临床实践中,我们预计这些研究成果将有助于开发对患者友好的治疗和诊断干预措施,包括自服和口服方案,从而改善 AD 患者的疾病管理和整体生活质量。此外,这项研究的范围超出了注意力缺失症,有可能为其他以蛋白质聚集为特征的神经退行性疾病提供启示。
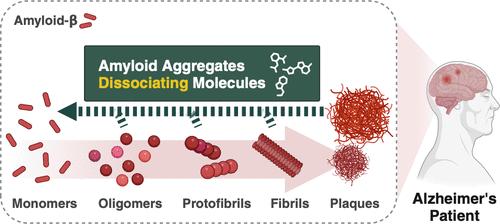
A century ago, German neurologist Alois Alzheimer documented the first case of Alzheimer's disease (AD), illuminating cognitive impairments associated with the presence of abnormal protein clusters, including amyloid plaques and tau tangles, within the brain. In a typical physiological state, the equilibrium of amyloid-β (Aβ) levels is maintained, but aging can precipitate disruptions in the homeostasis of Aβ due to its overproduction, impaired clearance, and other factors, ultimately leading to its accumulation. Although the link between Aβ aggregates and neurodegeneration has long made Aβ a promising target for AD, decades without successful drug development targeting Aβ have generated skepticism regarding the efficacy of this strategy for AD therapy. However, recent approvals of anti-Aβ antibody drugs by the FDA, including aducanumab (Aduhelm), lecanemab (Leqembi), and donanemab (Kisunla), have prompted a re-evaluation of this perspective. These therapies have demonstrated efficacy in reducing brain Aβ levels, thereby decelerating disease progression and reaffirming Aβ as a key target. Despite advancements, immunotherapies are accompanied by considerable disadvantages, including adverse effects, high costs, and cumbersome administration. To address these limitations, our research has focused on developing small molecules that can mitigate the challenges of antibody treatments while offering practical and accessible options. We identified 4-(2-hydroxyethyl)-1-piperazine propanesulfonic acid (EPPS) as a promising compound that significantly reduces aggregated Aβ in the brain and enhances behavior in AD rodent models. Following administration, EPPS penetrates the blood-brain barrier (BBB) and binds to toxic Aβ aggregates, subsequently breaking them down into nontoxic monomers. This leads to two significant outcomes: a reduction of Aβ aggregates in the brain and a subsequent increase in Aβ monomers in blood. The monomeric Aβ, unlike its aggregated form, can now traverse the BBB and enter the bloodstream. This mechanism provides an innovative approach to AD treatment and diagnosis. By detaching cerebral Aβ aggregates, EPPS facilitates Aβ clearance and addresses a key pathological feature of AD. Concurrently, the increase in blood Aβ levels offers a potential biomarker for monitoring treatment efficacy and disease progression, thereby revolutionizing both AD treatment and diagnosis. Investigating the detailed mode of action of drug candidates requires structural information about a target protein. Unfortunately, the unstable and heterogeneous nature of Aβ aggregates, which form larger clusters, complicates the identification of these structures. Therefore, we developed new tools for screening small molecules by immobilizing monomeric Aβ and its fragments on plates. This allows us not only to identify novel compounds that target Aβ but also to elucidate their mechanisms of action, enabling the development of Aβ-targeting therapeutic avenues in AD. We believe that our work on chemical-driven amyloid clearance through small molecules represents an advance in AD research, offering chemical diversity and straightforward, economical development processes. In clinical practice, we anticipate that these findings will contribute to the development of patient-friendly therapeutic and diagnostic interventions, including self-administered and orally available options, thereby enhancing disease management and overall quality of life for individuals with AD. Furthermore, this research extends beyond AD, potentially offering insights into other neurodegenerative diseases characterized by protein aggregation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


