逆转海恩斯中间体的反应性以简易合成取代的 3-羟基喹啉类化合物
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
一种高效、通用的 3-羟基喹啉类化合物合成方法已经实现,该方法由邻乙酰苯胺和 α-羟基酮合成,收率良好。该策略涉及原位生成的氨基烯醇中间体与亲电羰基的分子内反向捕获,即间断的海恩斯重排,然后进行芳香化。该方法的重要特点包括良好的官能团耐受性、操作简单、克级合成和广泛的合成用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
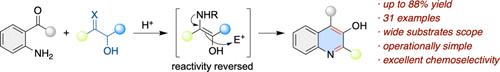
Reversal of Reactivity of Heyns Intermediate for the Concise Synthesis of Substituted 3-Hydroxyquinolines
An efficient and general method for the synthesis of 3-hydroxyquinolines has been achieved from o-acylanilines and α-hydroxyketones in good yields. The strategy involves the intramolecular reverse trapping of the in situ generated aminoenol intermediate with an electrophilic carbonyl, viz. an interrupted Heyns rearrangement, followed by aromatization. Important features include good functional group tolerance, operational simplicity, gram-scale synthesis, and broad synthetic utility.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


